|
Phoronida and Brachiopoda in the Indian River (Florida, USA)
Christian C. Emig
[Abstract - Résumé]
Version 
 by Google by Google
 mit Google mit Google
Introduction
Au cours de l’année 1980, de nombreux lots de Phoronida m’ont été envoyés pour identification suite à un programme sur la faune benthique dans l’Indian River et offshore en Floride (USA) (Fig. 1). Puis, un envoi complémentaire avait été effectué en 1981, contenant aussi des brachiopodes, venant de prélèvements fait offshore (Fig. 1). La condition pour réaliser leur identification était de pouvoir publier les résultats, ce à quoi R. Virnstein s’était engagé par lettre du 11-12-1980, qui stipulait: “I will be publishing the material from the Indian River as a series of descriptive ecological papers on the temporal and spatial distributions of all macrobenthos, and probably more detailed by taxonomic groups.” Il faut souligner que l’identification des phoronidiens se fait sur coupes histologiques, ce qui implique une logistique technique lourde et consommatrice de temps.
Une fois une partie des résultats envoyée, il s’est avéré qu’aucune publication concernant les Phoronida n’avait été prévue, mais que certains de mes résultats ont été utilisés et publiés en 1983 dans Florida Scientist. En conséquence, la collaboration avec les responsables du “Survey of the benthic fauna in the Indian River region”, menée par le “department of benthic ecology of the Harbor branch Foundation” (Fort-Pierce, Florida) a été rompue. Les collections ont été renvoyées à la Harbor branch Foundation sans mentionner tous les résultats des identifications faites et sans déterminer les brachiopodes.
A la retraite depuis une dizaine d’année, je viens de rouvrir ce dossier pour enfin publier les données complètes sur les Phoronida, après presque 40 ans. Un premier manuscrit avait été préparé pour une publication en 1981-1982, mais il n’a jamais été envoyé et mes résultats sur les phoronidiens et les brachiopodes de l’Indian River n’ont jamais été publiés. Ils sont reproduits, ci-dessous, en fac-similé ou scannés et traité par OCR (Optical Character Recognition) avec toutes les données complémentaires en ma possession. Une diagnose pour chaque espèce, à jour en 2019, est fournie.
Liste et localisation des stations
Station descriptions and locations of sites sampled by the Harbor branch benthic ecology department where Phoronida or Brachiopoda have been sampled (Fig. 1).
- Link Port (St. Lucie Co., FL) –
- St. 45-47: east and west of intracoastal channel markers 171-172; 27°32.1'N, 80°20.6'W, 91m north of the east end of the north peninsula of Link Port Channel;
St. 69-continuing: 13m from the west shore, 100m north of the north peninsula of Link Port Channel; 27°32.1'N, 80°21.0’W.
- Nuclear Power Plant (St. Lucie Co., FL) –
- west of Herman's Bay Point, Hutchinson Island ; both sides of the Indian River in line E-W with intracoastal channel marker 206; 27°20.5'N. 80°15.3'W for St. 48.
- Haulover Cove (Brevard Co., FL) –
- NW of the bridge over Haulover Canal ; north of the easterly end of the north spoil bank's northern shoreline, cove area, east side of the Indian River; 28°44.0’N, 80°45.5'W.
- Hog Point (Brevard Co., FL) –
- in line with intracoastal channel marker 23; east side of the Indian River; 27°58.7'N, 80°31.7’W
- St. Lucie (Martin Co., FL) –
- NW of Seminole Shores' NW seawall (now known Sallfish Point), east side of the Indian River; 27°11.0'N, 80°10.3'W.
- Drop Net Site (Indian River Co., FL) –
- 450 m west of Round Island, 300 m SW of the Round Island sample site, east side of the Indian River; 27°33.4'N, 80°20.6'W.
- Offshore (1) –
- R: I-III – st. 292/915-916, 294/923-924, 300/1098-1101: 25.4 km east of Ft. Pierce Inlet, depth 33-35 m; 27°33.2’N, 80°03.2'W.
- Offshore (2) –
- R: VII – st. 295, 299, 319: 36.1 km east of Ft. Pierce Inlet, depth 121 m, 27°28.6’N, 79°56.3'W. St. 323: 124 m, 27°29.7’N, 79°56.7'W. St. 327: 128 m, 27°29.5’N, 79°56.3'W.
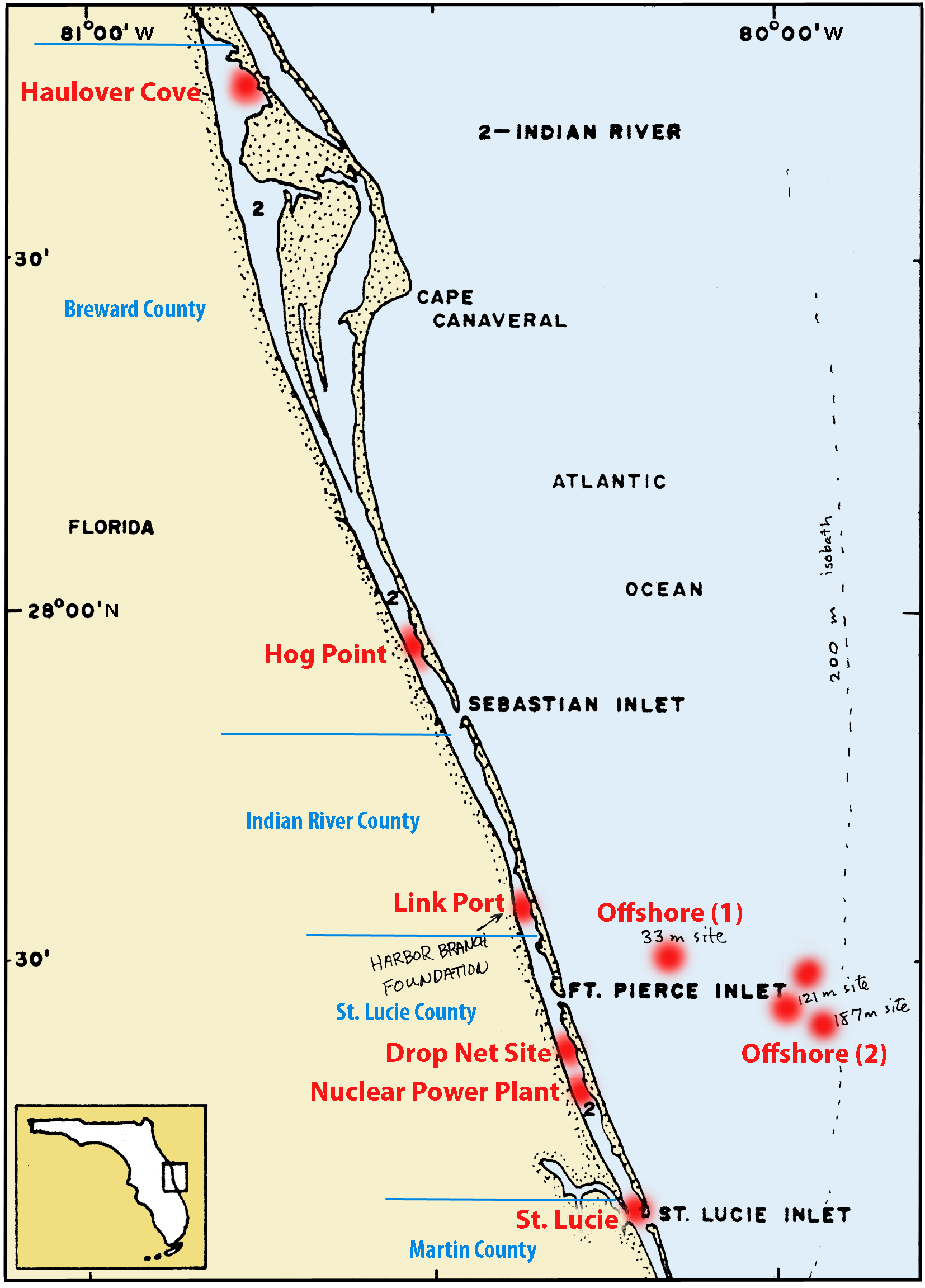
Fig. 1. – Localisation des stations dans Indian River (Floride) – voir texte et tables pour les détails.
A noter qu’une étude préliminaire citant des Phoronida a été réalisée dans Indian River Lagoon par Fehlmann (1974).
--- Beginning of the manuscript 1981 in facsimile or ocr --->>
Taxonomical part
Some taxonomic characteristics and their variations in the three phoronid species sampled during the Indian River survey will be improved within the diagnosis of each species, established by Emig (1979).
1. Phoronis psammophila Cori
P. psammophila, cosmopolitan, is a well-known species along the East coast of United States, and particularly on the Florida coast (cf. Emig, 1973, 1975, 1982a, 1982b).
The chitinous tube of this species is straight and covered by sand, grains. P. psammophila is found from the intertidal zone to about 35 m depth (this lowest depth has been recently cited by Emig, 1982b). The depth range of our specimens is from 0 to 33 m (Table 2; Fig. 1).
The extended size of P. psammophila reaches 190 mm in length while the diameter ranges from 0.5 to 2 mm. The trunk colour is pink; the lopho- phore is transparent with white spots, sometimes coloured in yellow, red or green. The lophophore is horseshoe-chaped with a number of tentacles up to 130; the tentacles range from 1.5 to 2.5 mm in length. Several individuals of our samples show a lophophore in diverse regeneration stages. The paired metanephridia opens into the trunk coelom by a single funnel; they show a descending and an ascending branch and open on either side of the anal papilla by the nephridiopore, below the anus. A single large nerve fibre (up to 27 µm in diameter) occurs in the trunk wall on the left side. The longitudinal muscle formulae of the studied specimens are given on table 2. Thus, the general composite formula of P. psammophila becomes now as following:
|
[25 - 53]
|
7-19⎪ 7-17
4-11 ⎪ 4-11 |
and the new mean formula of the species
|
35 =
|
12 ⎪11
6 ⎪ 6
|
P. psammophila is dioecious. The females broods their embryos in a single brood-mass by means of nidamental glands (Emig, 1977). The males have large and glandular lophophoral organs. Several specimens collected between 22 May and 24 August showed lophophoral organs or nidamental glands with mature tests or ovary. The larva of P. psammophila is Actinotrocha sabatieri (cf. Herrmann, 1979; Emig, 1982b).
2. Phoronis muelleri Selys-Longchamps
P. muelleri was recently recorded at Chesapeake Bay (Virginia) by Emig (1975, 1982a, 1982b), and at Beaufort (North Carolina) by Stancyk et al. (1976), in the type locality of P. psammophila. It is a cosmopolitan species characteristic of muddy bottoms and its bathymetric range varies from 0.6 to 390 m (Emig, 1982b), from 2 to 121 m in the studied stations herein (Table 3; Fig. 1). The tube is more or less straight cemented with sand grains. P. muelleri may reach about 12 cm in length, with a diameter of 0.2 to 1 mm. The body colour is yellowish to pink, occasionally the lophophore bears spots. The lophophore is horseshoe-shaped, but the tentacles become shorter in the middle of the oral side. The tentacles, about 1-2 mm in length, are up to 100 in number. Both nephridia bear an ascending and descending branch, ending by a single funnel in the trunk coelom and opening by the nephridiopore on the anal papilla at the anus level. There is a large conspicuous nerve fibre (up to 40 µm in diameter) on the left side. The left lateral mesentery lacks in P. muelleri that is indicated in the muscle formulae by a dotted line. The muscle formulae of the sampled specimens are given in Table 3. Herein the general composite formula of this species given by Emig (1979) is modified by the data from the present paper (Table 3) and samples during an expedition in the West Indian Ocean:
|
[18 - 39]
|
5-13 ⎪ 5-12
2- 8 ⎪ 3-8
|
and the mean formula of the species
|
26 =
|
8 ⎪9
4 ⎪4
|
P. muelleri is dioecious; males have large and glandular lophophoral organs and females lack nidamental glands and shed freely the ova into sea-water through the nephridia acting as gonoducts. The larva is Actinotrocha branchiata.
3. Phoronopsis harmeri Pixell
Up to the present time, the occurrence of this species in the Atlantic Ocean has been reported in the Azores (Emig, 1972), and here is the first record on the East coast of America. Phoronopsis harmeri, is embedded vertically in straight sand-encrusted tubes, in soft sediments (sands to mud, sometimes with a course fraction). The depth range is from the intertidal zone to 89 m: about 33 m in our offshore sample (Table 3).
The genus Phoronopsis is characterized by the presence of an epidermal-fold below the lophophore. The body length of Phoronopsis harmeri extends up to 220 mm, diameter 0.6-4 mm. The colour is greenish to pink, with tentacles sometimes whitish pigmented. The lophophore is spiral-coiled with 1.5 to 2 coils on each side; the tentacles reach 400 in number and 2-5 mm in length. The nephridia have two coelomic funnels (anal smaller, oral larger), a U-shaped tube, and open by the nephridiopore below the anus on the anal papilla in the collar invagination. The giant nerve fibre reaches 60 µm in diameter, a short right one occurs from nervous ganglion until the nephridial level. The muscular formula established on the single sampled specimens is given on Table 3. Thus, the general composite formula of Phoronopsis harmeri becomes now as following:
|
[75 - 145]
|
20-49⎪ 23-55
12-28 ⎪ 11-26
|
and the mean formula of the species
|
113 =
|
37 ⎪37
21 ⎪18
|
Phoronopsis harmeri is dioecious: large and membranous lophophoral organs in male; no brooding patterns in female with ova shed freely into sea-water. The larva is Actinotrocha harmeri.
Table 1. - Occurrence of Phoronis psammophila in the Indian River (Florida, USA) and offshore.
|
Locality
|
stations |
n.i.
|
bottom
|
Depth
(in m)
|
Range of water temperature
and salinity
|
|
Link Port
|
46 (A, E, F)
|
4
|
Subtidal mud
|
2.50
|
20.5° - 28-29.5 psu
|
|
135 (C)
|
1
|
Halodule wrightii
|
0.25
|
|
|
137 (D)
|
1
|
0.25
|
|
|
149 (B)
|
2
|
0.35
|
19-29°C - 20-34 psu
|
|
184 (A)
|
3
|
0.55
|
|
185 (A)
|
1
|
0.55
|
|
188 (B)
|
1
|
0.55
|
|
206 (B)
|
1
|
0.65
|
|
232 (B)
|
1
|
0.45
|
|
261 (A)
|
1
|
0.47
|
|
Haulover Cove
|
103 (E)
|
1
|
Halodule wrightii
|
0.35
|
15.5-33°C – 23-40 psu
|
|
144 (A-D)
|
12
|
0.30
|
|
204 (C)
|
6
|
0.27
|
|
214 (B)
|
5
|
0.38
|
|
218 (A, C)
|
2
|
0
|
|
222 (A,B)
|
4
|
0.07
|
|
226 (A)
|
1
|
0.45
|
|
230 (C)
|
3
|
0.47
|
|
234 (C)
|
12
|
0.41
|
|
238 (D)
|
12
|
0.38
|
|
242 (A-D)
|
25
|
0.32
|
|
246 (A-D)
|
9
|
0.25
|
|
259 (A,B,D)
|
5
|
0.32
|
|
270 (A,B,D)
|
2
|
0.24
|
|
278 (A-D)
|
5
|
|
|
282 (A,B)
|
3
|
0.14
|
|
287 (B)
|
2
|
0.04
|
|
291 (B)
|
1
|
0.19
|
|
St. Lucie
|
131 (A,D)
|
2
|
Halodule wrightii
|
0.60
|
18-30.5°C - 28-36 psu
|
|
163 (C)
|
1
|
0.47
|
|
181 (D)
|
1
|
0.60
|
|
198 (D)
|
1
|
0.37
|
|
219 (A)
|
1
|
0.50
|
|
223 (A,B)
|
1
|
0.25
|
|
231 (B)
|
1
|
0.47
|
|
260 (A)
|
1
|
0.21
|
|
298 (B)
|
1
|
0.50
|
|
Nuclear Power Plant
|
48 (B)
|
1
|
Halodule wrightii
|
0.65
|
|
|
Hog Point
|
56 (C)
|
1
|
Halodule wrightii
|
0.25
|
28.5°C - 35 psu
|
|
Drop net site
|
249 (C-D)
|
15
|
Subtidal mud
|
0.48
|
28-29.5°C - 32-36 psu
|
|
250 (B)
|
1
|
Halodule wrightii
|
0.73
|
|
252 (B)
|
3
|
Subtidal mud
|
0.44
|
|
253 (A)
|
2
|
0.44
|
|
262 (A,B)
|
5
|
0.40
|
|
263 (A, B)
|
3
|
0.40
|
|
266 (A, B)
|
3
|
0.47
|
|
Offshore (1)
|
292/916
|
1
|
subtidal shell hash sand, silt
|
33
|
|
|
unkown
|
314 (D)
|
1
|
|
|
|
Table 2. - Muscular formulae obtained on the specimens Phoronis psammophila in the Indian River and offshore (Florida, USA) – facsimile of the original table from 1981.
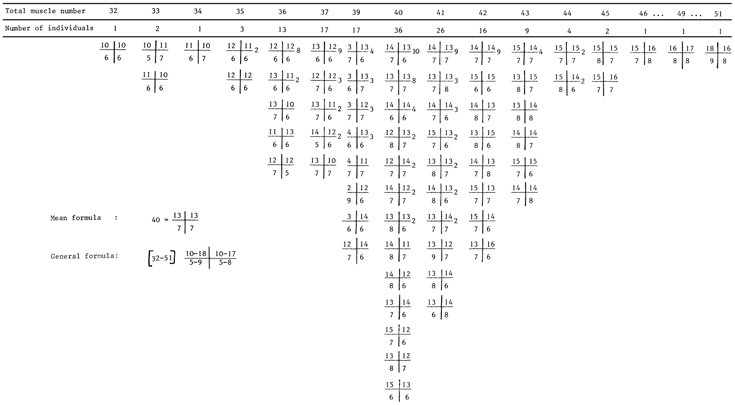
Table 3. - Occurrence of Phoronis muelleri and Phoronopsis harmeri in the Indian River (Florida, USA) and offshore (see location on Fig. 1).
|
Locality
|
station
|
n.i.
|
bottom
|
Depth (in m)
|
Species |
|
Link Port
|
46 (E,F)
|
2
|
Subtidal mud
|
2
|
Phoronis muelleri |
|
47 (C)
|
1
|
|
Offshore (2)
|
295 (A,B,C)
|
5
|
silt, sand shell hash
|
121
|
|
R-VII
|
1
|
mud
|
|
299 (A)
|
5
|
mud
|
|
Offshore (1)
|
292/916
|
1
|
shell hash sand, silt
|
33
|
Phoronopsis harmeri |
Longitudinal muscle formulae of the studied specimens of Phoronis muelleri (A) and Phoronopsis harmeri (B).
<< --- end of the manuscript in facsimile or ocr ----------
Données complémentaires
Phoronida :
Ultérieures au manuscrit ci-dessus, des données sur les phoronidiens ont été publiées dans le Florida Scientist par Hoskin (1983), Reish & Hallisey (1983), Virnstein et al. (1983) ; par Nelson & Virnstein (1995) ; lors de la « Indian River Lagoon Biodiversity Conference » (divers auteurs, 1995), éditée dans le Bulletin of Marine Science.
La description des biotopes (incluant les stations citées ici) a été publiée par Virnstein (1987) mais sans mention de phoronidiens. Dans la liste du « Indian River Lagoon Species Inventory » (Smithsonian Marine Station at Fort-Pierce, 2019), il y a mention des espèces suivants : Phoronis architecta [= synonyme de P. psammophila], P. hippocrepia, P. muelleri, Phoronis sp.
In the USA, two phoronid species are often quoted under the synonymous name (see also Emig, 2019):
- Phoronis architecta Brooks & Cowles, 1905 (instead of P. psammophila) is a case resolved by Emig (1977) when studying specimens from the architecta type locality in which two species occurred P. psammophila and P. muelleri that explains the original description by the two former authors with characters beared by one or other species according to the described specimens, and yet the original descriptions of both species have been quoted by Brooks & Cowles (1905).
- Phoronopsis vidiris Hilton, 1930 (instead of Phoronospsis harmeri) was proposed for synonymy by Marsden (1959), strengthened by Emig (1967), and confirmed by cladistic analysis (Emig, 1985). Forty-two years later, this synonymy was finally recognized and confirmed genetically as valid by Santagata & Cohen (2009).
La larve de P. psammophila (nommée P. architecta) a été récoltée par Jaeckle (2013) du quai de la « Smithsonian Marine Station » à Fort Pierce. Rappelons que cette larve s’appelle Actinotrocha sabatieri (voir Emig, 1982b ; Nielsen, 2013).
Brachiopoda :
The specimens could not be formally identified at specific level because their size is too small for (less than about 0.8-1 cm; see Emig, 1988). Nevertheless, they probably belong to Glottidia pyramidata which has been recorded is similar bottoms along the coast of Florida. Their distribution is given on Fig. 1 and Table 4.
Table 4. - Occurrence of brachiopods off the Indian River (Florida, USA) – see Fig. 1. The specimens were not identified.
|
Locality
|
station
|
bottom
|
Depth (in m)
|
Range of water temperature
and salinity
|
|
Offshore (1)
|
R : I-1,2
|
Subtidal silt, sand, shell hash
|
35
|
|
|
R : II-2,4,5
|
33
|
|
R : III-3,7
|
33
|
|
G300/1098
|
33
|
9-10.6°C – 36 psu
|
|
G300/1101
|
33
|
|
G292/915
|
33
|
|
|
G292/916
|
33
|
|
Offshore (2)
|
G294/923
|
187
|
|
SM294/924
|
|
319 (A, B)
|
115
|
|
323 (A)
|
124
|
|
327 (A)
|
128
|
Diagnoses of the Phoronida species (adults)
Les diagnoses des formes adultes des trois espèces de Phoronida, citées dans ce travail, sont reproduites d’après Emig (2019).
Phoronis psammophila Cori, 1889
Larva: Actinotrocha sabatieri Roule, 1890
Synonyms:
Phoronis sabatieri Roule, 1890
Phoronis architecta Andrews, 1890
? Phoronis euxinicola Selys-Longchamps, 1907
Diagnosis (from Emig, 2019)
Last update: July 22, 2006
Extended specimens up to 190 mm long, diameter 0.5-2 mm.
Colour in life: body pink, lophophore transparent with white (occasionally yellow, green or red) pigment spots.
Lophophore horseshoe-shaped with ends turned medially. Up to 190 tentacles, length 1.5-2.5 mm.
Nephridia with a single funnel, with descending and ascending branches; nephridiopore on anal papilla opening below anus.
Single giant nerve fibre on left side, 7-27 µm in diameter; very thin nerve fibre rarely present on right side.
Longitudinal muscle bundles of feathery type; the mean formula and general formula are respectively:
12 | 11
6 | 6
|
= 35
|
and
|
7-19 | 7-18
4-11 | 4-11
|
[24 - 53]
|
(n = 3244 individuals)
|
Sexual reproduction dioecious; females brooding embryos in a single mass in lophophoral cavity through nidamental glands of type C (i.e., formed by fusion of inner row of lophophore tentacles); males with large, glandular lophophoral organs.
Asexual reproduction by transverse fission.
Additional data:
Phoronis psammophila lives in the soft substratum at depths ranging from the intertidal zone to 69 m, commonly between 0 and 10 m (Fig. 2). Densities may reach 18,000 to 20,000 ind.m-2. This species particularly prefers a sediment structure mainly composed of fine and very fine sand fractions (58 to 93%), with a moderate muddy fraction (8 to 41%).
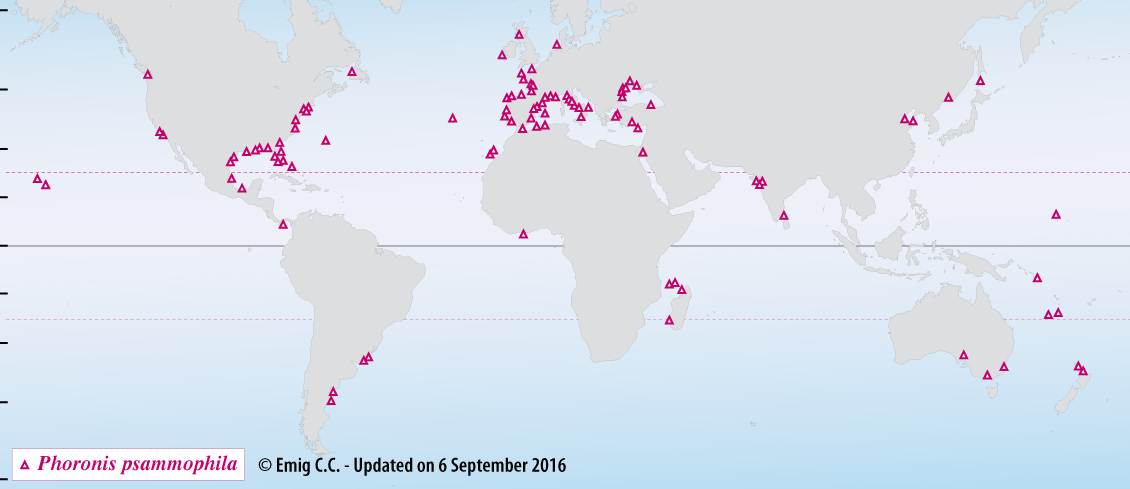
Fig. 2. – Distribution of Phoronis psammophila, a cosmopolitan species - Type-locality: Messina (Italy).
Phoronis muelleri Selys-Longchamps, 1903
Larva: Actinotrocha branchiata Müller, 1846
Diagnosis (from Emig, 2019)
Last update: June 24, 2004
Extended specimens up to 120 mm in length, 0.2-1 mm in diameter.
Colour in life: body pink and lophophore transparent, occasionally with spots.
Lophophore horseshoe-shaped with ends sometimes turned medially, but with the tentacles becoming shorter in the middle of the oral side. Number of tentacles up to 100, length 1-2 mm.
Nephridia opening into both oral and anal compartments of metacoelom by a single funnel, with descending and ascending branches ending by the nephridiopore on anal papilla, opening at level of anus.
A single giant nerve fiber on left side, 7-40 µm in diameter; a very thin nerve fiber rarely present on right side.
Left lateral mesentery absent except at level of left nephridium (indicated by a dotted line in the muscle formulae - but herein by the absence of line).
Longitudinal muscle bundles of feathery type; the mean formula and general formula are respectively:
9 | 9
4 | 4
|
= 26
|
and
|
5-13 | 5-12
2-8 | 2-8
|
[17 - 39]
|
(n = 620 individuals)
|
Sexual reproduction dioecious; females shed ova freely into the seawater; males have large, glandular lophophoral organs.
Asexual reproduction by transverse fission.
Additional data:
Phoronis muelleri penetrates vertically into muddy to sandy sediments with a high organic content and often with coarse particles of detritus and in the water column suspended material. The vertical distribution extends from the intertidal zone to about 400 m, but is mainly between 10 and 60 m (Fig. 3). Densities may reach ca. 3,000 ind.m-2. P. muelleri is frequently recorded in Macoma and Amphiura communities.
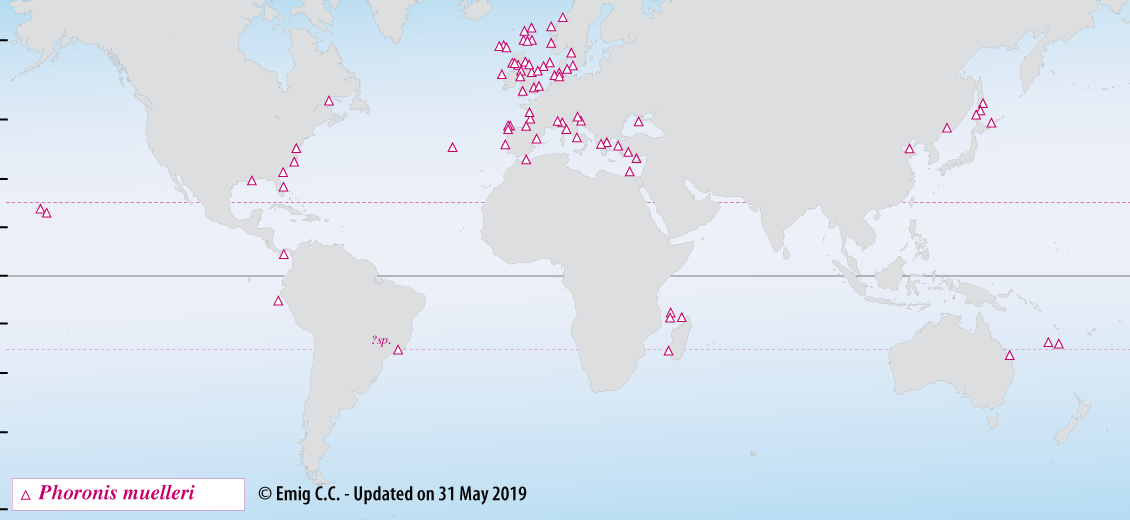
Fig. 3. – Distribution of Phoronis muelleri, a cosmopolitan species - Type-locality: Helgoland (Germany).
Phoronopsis harmeri Pixell, 1912
Larva: Actinotrocha harmeri Zimmer, 1964
Synonyms:
Phoronis pacifica Torrey, 1901
Phoronopsis striata Hilton, 1930
Phoronopsis viridis Hilton, 1930
? Phoronopsis malakhovi Temereva, 2000
Diagnosis (from Emig, 2019)
Last update: July 5, 2007
Collar-fold below the lophophore (genus character): well-marked around the lophophore.
Extended specimens up to 220 mm long, diameter 0.6-4 mm.
Colour in life: body pink to greenish, lophophore transparent sometimes white pigmented.
Lophophore spiral with 1 to 2.5 coils on each side. Up to 400 tentacles, 2-5 mm in length.
Nephridia with two pseudo-funnels (anal smaller, oral larger), descending and ascending branch, nephridiopore on anal papilla opening below anus on collar fold within invagination.
Two giant nerve fibres: left fibre: 20-60 µm in diameter; right fibre only present at the nephridial level.
Longitudinal muscle bundles of feathery type; the mean formula and general formula are respectively:
35 | 36
20 | 18
|
= 109
|
and
|
22-58 | 21-55
12-28 | 11-26
|
[75-166]
|
(n = 529 individuals)
|
Sexual reproduction dioecious; females shed the ova directly in the sea-water; males with large membranous lophophoral organs.
Asexual reproduction by transverse fission.
Additional data:
Phoronopsis harmeri is embedded vertically in soft sediments from sands to muddy sand, sometimes with a coarse fraction; depths range from the intertidal zone to 102 m, with a common range from 0-20 m (Fig. 4). Densities may reach 28,000 ind.m-2.
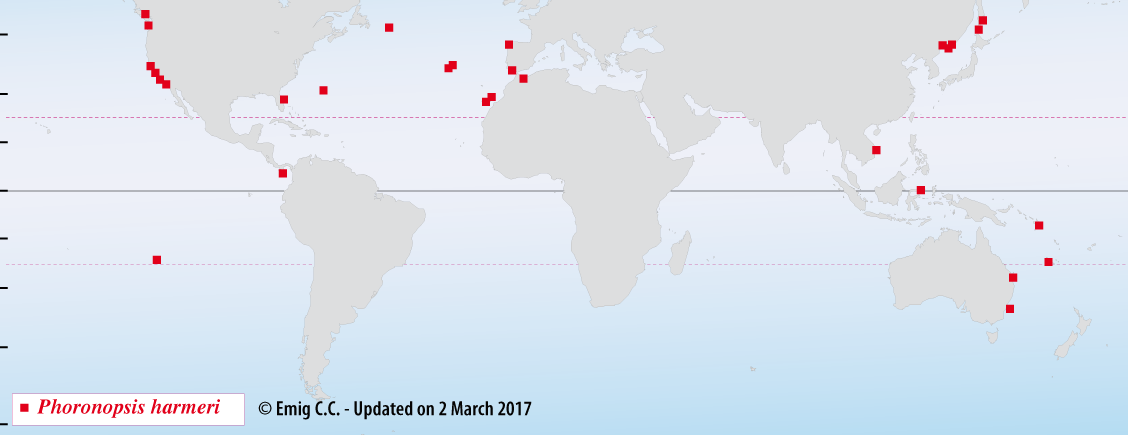
Fig. 4. – Distribution of Phoronopsis harmeri, a rather cosmopolitan species - Type-locality: Departure Bay, Vancouver Island (Canada).
Références
Brooks W. K. & R. P. Cowles, 1905. Phoronis architecta: its life history, anatomy and breeding habits. Mem. Nat. Acad. Sci. Washington, 10 (4), 72-113.
Divers auteurs (1995). Indian River Lagoon Biodiversity Conference. Bulletin of Marine Science, 57 (1), 300 p.
Emig C. C., 1967. Considérations sur la systématique des Phoronidiens. II. Phoronopsis harmeri Pixell, 1912. Bulletin du Muséum national d’Histoire naturelle de Paris, 39, 984-991.
Emig C. C., 1972. Phoronidiens récoltés lors de la campagne "Biaçores" du N/O Jean Charcot (3 octobre-20 novembre 1971). Téthys, 4, 423-428.
Emig C. C., 1973. Ecologie des Phoronidiens. Bulletin d’Ecologie, Paris, 4 (4), 339-364.
Emig C. C., 1975 (1977). Notes sur la localisation, l'écologie et la taxonomie des Phoronidiens. Téthys, 7 (4), 357-364.
Emig C. C., 1977. Embryology of Phoronida. American Zooologist, 17 (1), 21-38.
Emig C. C., 1979. British and other Phoronids. In : Synopses of the British fauna, Eds Kermack D. M. & R. S. K. Barnes, Academic Press, London, 13, 57 p.
Emig C. C., 1982a. Nouvelles localisations de Phoronidiens. Téthys, 10 (3), 287-290.
Emig C. C., 1982b. The biology of Phoronida. Advances in marine Biology, 19, 1-89.
Emig C. C., 1983. Taxonomie du genre Glottidia (Brachiopodes, Inarticulés). Bulletin du Muséum national d’Histoire naturelle de Paris, (4) 5 (Sect.4) (2), 469-489.
Emig C. C., 1985. Phylogenetic systematics in Phoronida (Lophophorata). Zeitschrift für zoologische Systematik und Evolution-Forschung, 23 (3), 184-193.
Emig C. C., 2019. Phoronida database. http://paleopolis.es/Phoronida_database/, accessed on 31-5-2019.
Fehlmann A. D., 1974. Indian River Study : a report on research and progress ending October 1974. https://fau.digital.flvc.org/islandora/object/fau%3A2434/datastream/OBJ/view
Herrmann K., 1979. Larvalentwicklung und Metamorphose von Phoronis psammophila (Phoronida, Tentaculata). Helgoländer wissenschaftliche Meeresuntersuchungen, 32, 550-581.
Hoskin C. M., 1983. Sediment in seagrasses near Link Port, Indian River, Florida. Florida Scientist, 46 (3/4), 153-161.
Jaeckle W, 2013. Seawater flow into the digestive system of actinotroch larvae (Phoronida). Annual Meeting for the Society for integrative and comparative Biology, San Francisco, 2013, http://works.bepress.com/jaeckle/48, consulté le 2 mai 2019.
Marsden J. R., 1959. Phoronidea from the Pacific coast of North America. Canadian Journal of Zoology, 37 (2), 87-111.
Nelson W. & R. Virnstein, 1995. Long-term dynamics of seagrass macrobenthos: asynchronous population variability in space and time. Proceedings of the 28th European Marine Biological Symposium, Crete, 1993. International Symposium Series, Olsen & Olsen, Copenhagen, p. 185-190.
Nielsen C., 2013. Phoronis Wright, 1856 (Phoronida) and P. muelleri Selys-Longchamps, 1903: proposed conservation of both names. Bulletin of Zoological Nomenclature, 70 (3), 157-159.
Reish D. J. & M. L. Hallisey, 1983. A check-list of the benthic macroinvertebrates of Kennedy space center, Florida. Florida Scientist, 46 (3/4), 306-313.
Santagata S. & B. L. Cohen, 2009. Phoronid phylogenetics (Brachiopoda; Phoronata): evidence from morphological cladistics, small and large subunit rDNA sequences, and mitochondrial cox1. Zoological Journal of the Linnean Society, London, 157, 34-50.
Smithsonian marine station at Fort-Pierce, 2019. Indian River Lagoon species inventory. Alphabetized species listing: P Species. https://naturalhistory2.si.edu/smsfp/irlspec/Pspecies1.htm, consulté le 2 mai 2019.
Virnstein R. W., 1987. Seagrass-associated invertebrate communities of the southeastern USA: a review. Florida Marine Research Publications, 42, 89-116.
Virnstein R.W., Mikkelsen P.S., Cairns K.D. & M.A. Capone, 1983. Seagrass beds versus sand bottoms: The trophic importance of their associated benthic invertebrates. Florida Scientist, 46 (3/4), 363-381.
|