![]() In his book, Life on a young planet,
A.H. states that the first documented fossils of green algae date back 750 Ma. However, according to B. 's
book, La vie invisible, they are much older.
Using a method which combines paleontology and molecular phylogeny, this paper is an inquiry into the Precambrian fossils of some "acritarchs" and of a
primitive clade of green algae, the Pyramimonadales. A paraphyletic group of unicellular green algae, named "Prasinophyceae", is represented at Thule (Greenland) ca. 1200 Ma by several morphotypes of the
monophyletic Pyramimonadales, including Tasmanites and Pterospermella
that are akin to algae still living today. These two, and others, probably had forerunners going back 1450 / 1550 Ma. Some acritarchs that may
represent Pyramimonadales producing "phycomas" which split open for dehiscence were
confusingly included in the polyphyletic pseudo-taxon "Leiosphaeridia" and are possibly already present at Chuanlinggou, China, ca. 1730 Ma. Many
acritarchs that obtained by acid maceration of Russian samples dated between 1800 and 2000 Ma were probably unicellular Chlorophyta which
synthesized algaenans or other biopolymers resistant to acetolysis.
Living Prasinophyceae are undoubtedly green algae (Viridiplantae). Thus, if Prasinophyceae fossils go back
certainly to 1200 Ma, probably to 1500 Ma and possibly to 1730 Ma, then the ancestor of green algae (Chlorophyta and Streptophyta) probably separated from the ancestor of red algae (Rhodophyta) as early as 2000
Ma.
In his book, Life on a young planet,
A.H. states that the first documented fossils of green algae date back 750 Ma. However, according to B. 's
book, La vie invisible, they are much older.
Using a method which combines paleontology and molecular phylogeny, this paper is an inquiry into the Precambrian fossils of some "acritarchs" and of a
primitive clade of green algae, the Pyramimonadales. A paraphyletic group of unicellular green algae, named "Prasinophyceae", is represented at Thule (Greenland) ca. 1200 Ma by several morphotypes of the
monophyletic Pyramimonadales, including Tasmanites and Pterospermella
that are akin to algae still living today. These two, and others, probably had forerunners going back 1450 / 1550 Ma. Some acritarchs that may
represent Pyramimonadales producing "phycomas" which split open for dehiscence were
confusingly included in the polyphyletic pseudo-taxon "Leiosphaeridia" and are possibly already present at Chuanlinggou, China, ca. 1730 Ma. Many
acritarchs that obtained by acid maceration of Russian samples dated between 1800 and 2000 Ma were probably unicellular Chlorophyta which
synthesized algaenans or other biopolymers resistant to acetolysis.
Living Prasinophyceae are undoubtedly green algae (Viridiplantae). Thus, if Prasinophyceae fossils go back
certainly to 1200 Ma, probably to 1500 Ma and possibly to 1730 Ma, then the ancestor of green algae (Chlorophyta and Streptophyta) probably separated from the ancestor of red algae (Rhodophyta) as early as 2000
Ma.
![]() Chlorophyta, Leiosphaeridia, Prasinophyceae, Precambrian algae,
Pterospermella, Pterospermopsimorpha, Pyramimonadales, Spiromorpha, Tasmanites, Viridiplantae.
Chlorophyta, Leiosphaeridia, Prasinophyceae, Precambrian algae,
Pterospermella, Pterospermopsimorpha, Pyramimonadales, Spiromorpha, Tasmanites, Viridiplantae.
B. (2006).- Are the green algae (phylum Viridiplantae) two billion years old?.- Carnets de Géologie / Notebooks on Geology, Brest, Article 2006/03 (CG2006_A03)
![]() Les algues vertes (phylum Viridiplantae) sont-elles vieilles de deux milliards d'années
?.- Dans son livre, Life on a young planet, A.H.
prétend que les plus anciens fossiles bien attestés d'algues vertes remontent
à 750 Ma. Cependant, selon le livre de B. , La vie invisible,
certains d'entre eux sont beaucoup plus vieux. L'article qui suit recourt à une
méthode qui combine la paléontologie et la phylogénie moléculaire pour une
recherche sur les fossiles précambriens de quelques "acritarches" et
d'un clade primitif d'algues vertes, les Pyramimonadales. Un assemblage
paraphylétique d'algues vertes unicellulaires, appelé
"Prasinophyceae", est représenté vers 1200 Ma dans le Supergroupe de
Thulé, au Groenland, par plusieurs morphotypes des Pyramimonadales, qui sont
monophylétiques, en particulier Tasmanites et Pterospermella
apparentés à des algues encore vivantes aujourd'hui. Ces deux genres, parmi
d'autres, ont eu probablement des précurseurs vers 1450 / 1550 Ma. Certains
acritarches qui pourraient représenter des Pyramimonadales produisant des
"phycomes" qui s'ouvraient par une fissure lors de leur déhiscence
ont été confusément rassemblés dans le pseudo-taxon polyphylétique "Leiosphaeridia".
Ils étaient peut-être déjà présents à Chuanlinggou, en Chine, vers 1730
Ma. Beaucoup d'acritarches de Russie que a
obtenus par macération dans l'acide fluorhydrique et qui ont été datés entre
1800 et 2000 Ma représentent probablement des Chlorophytes unicellulaires
capables de synthétiser des algaenanes ou autres biopolymères résistants à
l'acétolyse. Les Prasinophyceae qui vivent encore de nos jours sont
incontestablement des algues vertes (Viridiplantae). Par conséquent, s'il est
vrai que les fossiles de Prasinophyceae remontent certainement à 1200 Ma,
probablement à 1500 Ma et peut-être même à 1730 Ma, il faut conclure que
l'ancêtre des algues vertes (Chlorophyta et Streptophyta) s'était probablement
déjà séparé de l'ancêtre des algues rouges (Rhodophyta) à une date proche
de 2000 Ma.
Les algues vertes (phylum Viridiplantae) sont-elles vieilles de deux milliards d'années
?.- Dans son livre, Life on a young planet, A.H.
prétend que les plus anciens fossiles bien attestés d'algues vertes remontent
à 750 Ma. Cependant, selon le livre de B. , La vie invisible,
certains d'entre eux sont beaucoup plus vieux. L'article qui suit recourt à une
méthode qui combine la paléontologie et la phylogénie moléculaire pour une
recherche sur les fossiles précambriens de quelques "acritarches" et
d'un clade primitif d'algues vertes, les Pyramimonadales. Un assemblage
paraphylétique d'algues vertes unicellulaires, appelé
"Prasinophyceae", est représenté vers 1200 Ma dans le Supergroupe de
Thulé, au Groenland, par plusieurs morphotypes des Pyramimonadales, qui sont
monophylétiques, en particulier Tasmanites et Pterospermella
apparentés à des algues encore vivantes aujourd'hui. Ces deux genres, parmi
d'autres, ont eu probablement des précurseurs vers 1450 / 1550 Ma. Certains
acritarches qui pourraient représenter des Pyramimonadales produisant des
"phycomes" qui s'ouvraient par une fissure lors de leur déhiscence
ont été confusément rassemblés dans le pseudo-taxon polyphylétique "Leiosphaeridia".
Ils étaient peut-être déjà présents à Chuanlinggou, en Chine, vers 1730
Ma. Beaucoup d'acritarches de Russie que a
obtenus par macération dans l'acide fluorhydrique et qui ont été datés entre
1800 et 2000 Ma représentent probablement des Chlorophytes unicellulaires
capables de synthétiser des algaenanes ou autres biopolymères résistants à
l'acétolyse. Les Prasinophyceae qui vivent encore de nos jours sont
incontestablement des algues vertes (Viridiplantae). Par conséquent, s'il est
vrai que les fossiles de Prasinophyceae remontent certainement à 1200 Ma,
probablement à 1500 Ma et peut-être même à 1730 Ma, il faut conclure que
l'ancêtre des algues vertes (Chlorophyta et Streptophyta) s'était probablement
déjà séparé de l'ancêtre des algues rouges (Rhodophyta) à une date proche
de 2000 Ma.
![]() Algues précambriennes,
Chlorophytes, Leiosphaeridia, Prasinophyceae, Pterospermella, Pterospermopsimorpha, Pyramimonadales,
Spiromorpha, Tasmanites, Viridiplantae.
Algues précambriennes,
Chlorophytes, Leiosphaeridia, Prasinophyceae, Pterospermella, Pterospermopsimorpha, Pyramimonadales,
Spiromorpha, Tasmanites, Viridiplantae.
![]() The antiquity
of the green algae (Viridiplantae) has been hotly debated and is still
controversial today. In his book, Life on a young planet
(2003),
Andrew H. published a diagram (p. 152, fig.
9.5) assigning to the first documented fossils of these algae an age of 750
million years (Ma) and to the separation of their ancestors from those of the
red algae a date somewhat more than 1200 Ma. On the contrary, in my book, La vie invisible
(, 2002),
I argue that the oldest known fossils of green algae date back at least to 1200
Ma, and that the ancestors of Viridiplantae and Rhodophyta separated from each
other perhaps as early as 2000 Ma. The matter is of consequence and the
disagreement obvious.
The antiquity
of the green algae (Viridiplantae) has been hotly debated and is still
controversial today. In his book, Life on a young planet
(2003),
Andrew H. published a diagram (p. 152, fig.
9.5) assigning to the first documented fossils of these algae an age of 750
million years (Ma) and to the separation of their ancestors from those of the
red algae a date somewhat more than 1200 Ma. On the contrary, in my book, La vie invisible
(, 2002),
I argue that the oldest known fossils of green algae date back at least to 1200
Ma, and that the ancestors of Viridiplantae and Rhodophyta separated from each
other perhaps as early as 2000 Ma. The matter is of consequence and the
disagreement obvious.
![]() I have the
greatest admiration for A.H. , whom I consider
one of the most distinguished specialists of the Precambrian. I do not contest
the facts he reported (with one significant exception: I maintain that some
green algae, the Pyramimonadales, were attested long before 750 Ma). Several of
the data I use come from 's papers. The
disagreement mainly concerns two points, one theoretical, the other
methodological.
I have the
greatest admiration for A.H. , whom I consider
one of the most distinguished specialists of the Precambrian. I do not contest
the facts he reported (with one significant exception: I maintain that some
green algae, the Pyramimonadales, were attested long before 750 Ma). Several of
the data I use come from 's papers. The
disagreement mainly concerns two points, one theoretical, the other
methodological.
![]() 1) I disagree
with the whole theory underlying the "short chronology" that assumed in preparing diagram 9.5 of his book, Life on a young planet
(p. 152). This diagram looks to me like an attempt to reconcile some of the
phylogenetic trees of the Eukaryotes (ibid., p. 127, fig. 8.2) with the
hypothesis of an "evolutionary big bang" that presented in a famous 1992 paper.
According to this hypothesis the radiation of the "crown-group" of
Eukaryotes was explosive. It was induced by a rapid increase in atmospheric
oxygen between 1200 and 1000 Ma. Since 1992
has nuanced his theory with many shades and derogations. However, to my
knowledge, he has never formally stated that he may have been wrong. Concerning
this topic I defend in my book, La vie invisible
(, 2002),
three ideas. The first two are in agreement with 's
concepts, whereas the third is radically contrary:
1) I disagree
with the whole theory underlying the "short chronology" that assumed in preparing diagram 9.5 of his book, Life on a young planet
(p. 152). This diagram looks to me like an attempt to reconcile some of the
phylogenetic trees of the Eukaryotes (ibid., p. 127, fig. 8.2) with the
hypothesis of an "evolutionary big bang" that presented in a famous 1992 paper.
According to this hypothesis the radiation of the "crown-group" of
Eukaryotes was explosive. It was induced by a rapid increase in atmospheric
oxygen between 1200 and 1000 Ma. Since 1992
has nuanced his theory with many shades and derogations. However, to my
knowledge, he has never formally stated that he may have been wrong. Concerning
this topic I defend in my book, La vie invisible
(, 2002),
three ideas. The first two are in agreement with 's
concepts, whereas the third is radically contrary:
![]() a) the expansion
of Eukaryotes correlates closely with the increase in atmospheric oxygen;
a) the expansion
of Eukaryotes correlates closely with the increase in atmospheric oxygen;
![]() b) the effects of
this increase did not occur as a continuous progression, but as successive
stages separated by thresholds;
b) the effects of
this increase did not occur as a continuous progression, but as successive
stages separated by thresholds;
![]() c) the phase that
stimulated the expansion of the Eukaryotes did not occur between 1200 and 1000
Ma, as (1992)
asserted, but considerably earlier, during a period separating two well-defined
Huronian glaciations around 2400 Ma.
c) the phase that
stimulated the expansion of the Eukaryotes did not occur between 1200 and 1000
Ma, as (1992)
asserted, but considerably earlier, during a period separating two well-defined
Huronian glaciations around 2400 Ma.
![]() 2) The second
disagreement is a methodological one. It seems to me that it is now essential to
confront paleontology with a science that is developing exponentially today:
molecular phylogeny. In his most recent publications
accepts this concept in a general way, but in my opinion he does not draw all
the inferences from the fact that a paleontologist must master those aspects of
this science that concern phylogenetic trees both as a method for control and as
a heuristic tool. On the one hand, although molecular phylogeny does not always
allow us to establish an exact taxonomy, it helps us to avoid gross errors due
to homoplasy or morphological convergence. If a paleontologist avoids this
interdisciplinary collation, he lays himself open to a misinterpretation of the
phylogenetic relationships of the fossils. Furthermore, molecular phylogeny
allows us to determine the sequence of nodal points, the order of the successive
evolutionary stages in a phylum. This sequence is an important criterion for
evaluating the probability that a fossil discovered at a given geological level
does or does not belong to a given taxonomic clade. The reader may find in the
annexes of this paper two examples of the usefulness of molecular phylogeny as a
means of detecting false assertions (,
2006, annex 15) or as a heuristic
tool (, 2006,
annex 16).
2) The second
disagreement is a methodological one. It seems to me that it is now essential to
confront paleontology with a science that is developing exponentially today:
molecular phylogeny. In his most recent publications
accepts this concept in a general way, but in my opinion he does not draw all
the inferences from the fact that a paleontologist must master those aspects of
this science that concern phylogenetic trees both as a method for control and as
a heuristic tool. On the one hand, although molecular phylogeny does not always
allow us to establish an exact taxonomy, it helps us to avoid gross errors due
to homoplasy or morphological convergence. If a paleontologist avoids this
interdisciplinary collation, he lays himself open to a misinterpretation of the
phylogenetic relationships of the fossils. Furthermore, molecular phylogeny
allows us to determine the sequence of nodal points, the order of the successive
evolutionary stages in a phylum. This sequence is an important criterion for
evaluating the probability that a fossil discovered at a given geological level
does or does not belong to a given taxonomic clade. The reader may find in the
annexes of this paper two examples of the usefulness of molecular phylogeny as a
means of detecting false assertions (,
2006, annex 15) or as a heuristic
tool (, 2006,
annex 16).
![]() In the last
thirty years paleontological analysis of the Prasinophyceae has been
reinvigorated, for morphological description of fossils is now supplemented by
observation of cell ultrastructures using the TEM microscope and by biochemical
analysis of the cellular wall. Eminent paleontologists distinguish themselves in
these fields, following the pioneer work of , , , , , , , , , .
Since 1995 several researchers have insisted explicitly as a principle on the
necessity of combining ultrastructural and biochemical analyses with traditional
descriptions. See for instance et alii (1999, 2000),
& (2000),
& (2004),
, & (2001,
2004),
et alii (2005).
It seems to me that the time has come to enter a new phase, that would be the
third one, in which a paleontology that aims at becoming explanatory and not
merely descriptive would integrate its findings with those of molecular
phylogeny.
In the last
thirty years paleontological analysis of the Prasinophyceae has been
reinvigorated, for morphological description of fossils is now supplemented by
observation of cell ultrastructures using the TEM microscope and by biochemical
analysis of the cellular wall. Eminent paleontologists distinguish themselves in
these fields, following the pioneer work of , , , , , , , , , .
Since 1995 several researchers have insisted explicitly as a principle on the
necessity of combining ultrastructural and biochemical analyses with traditional
descriptions. See for instance et alii (1999, 2000),
& (2000),
& (2004),
, & (2001,
2004),
et alii (2005).
It seems to me that the time has come to enter a new phase, that would be the
third one, in which a paleontology that aims at becoming explanatory and not
merely descriptive would integrate its findings with those of molecular
phylogeny.
![]() I am currently
working on research which could be entitled "Precambrian paleontology in
the light of molecular phylogeny". The object of the study presented here
is to demonstrate the antiquity of the Viridiplantae, by focusing on fossils
that probably pertain to one of the divisions of this phylum, the Chlorophyta,
and more particularly to the clade of the Pyramimonadales. I shall defend two
proposals:
I am currently
working on research which could be entitled "Precambrian paleontology in
the light of molecular phylogeny". The object of the study presented here
is to demonstrate the antiquity of the Viridiplantae, by focusing on fossils
that probably pertain to one of the divisions of this phylum, the Chlorophyta,
and more particularly to the clade of the Pyramimonadales. I shall defend two
proposals:
- The oldest known fossils of green algae do not date back to circa 750 Ma, as indicated in 's diagram (2003, p. 152), but at least to 1200 Ma and probably much earlier.
- The divergence of green and red algae did not occur slightly before 1200 Ma, but about 2000 Ma.
![]() The arguments
I develop have been set out in nine headings, so that A.H.
may, if he likes, clearly identify them in his counter-arguments. A series of
documentary papers complementing my discussion can be found in the annexes (,
2006, annexes 1-16).
The arguments
I develop have been set out in nine headings, so that A.H.
may, if he likes, clearly identify them in his counter-arguments. A series of
documentary papers complementing my discussion can be found in the annexes (,
2006, annexes 1-16).
![]() 1. The
phylogeny and taxonomy of the Chlorophyta, particularly those of the
"Prasinophyceae", have been deeply modified by the results of
molecular analysis during the last ten years (see , 2006,
annex 1).
1. The
phylogeny and taxonomy of the Chlorophyta, particularly those of the
"Prasinophyceae", have been deeply modified by the results of
molecular analysis during the last ten years (see , 2006,
annex 1).
![]() The phylum
Viridiplantae comprises two sections: the Chlorophyta, commonly divided into the
"classes" Prasinophyceae, Chlorophyceae, Trebouxiophyceae and
Ulvophyceae, and the Streptophyta that regroup the paraphyletic series of the
Charophyceae and the terrestrial plants, or Embryophyta (see , 2006,
annexes 1-2 and 12). According to most phylogenetic trees the phylum of the
Viridiplantae first joined that of the Rhodophyta, then both joined that of the
Glaucophyta, and together they form the "kingdom of Plantae" ,
1981.
The phylum
Viridiplantae comprises two sections: the Chlorophyta, commonly divided into the
"classes" Prasinophyceae, Chlorophyceae, Trebouxiophyceae and
Ulvophyceae, and the Streptophyta that regroup the paraphyletic series of the
Charophyceae and the terrestrial plants, or Embryophyta (see , 2006,
annexes 1-2 and 12). According to most phylogenetic trees the phylum of the
Viridiplantae first joined that of the Rhodophyta, then both joined that of the
Glaucophyta, and together they form the "kingdom of Plantae" ,
1981.
![]() When
asserts that the oldest known fossils of green algae date back to 750 Ma, he is
presumably thinking of the multicellular green algae that belong to the two
clades of the Chlorophyta that nowadays are the most abundant and diverse, i.e.
the Chlorophyceae and the Ulvophyceae. For an unknown reason he does not
consider the "Prasinophyceae", although they are indisputably
unicellular green algae. Chlorophyceae and Ulvophyceae stand at the top of the
phylogenetic tree of Chlorophyta. They may be called "Neochlorophyta" (see , 2006,
annex 2), meaning that they are preceded by a very long history. Several sites
dated between circa 640 Ma (start of the Varanger glaciation) and circa 770 Ma
have yielded fossils attributed to Dasycladales (Biskopås in Norway: , 1990),
to Ulotrichales (Skillogale in Australia: , 1977),
or more indisputably to Siphonocladales and Sphaeropleales (Svanbergfjellet in
Spitzbergen: et alii, 1994).
Older specimens, dated about 850 Ma, have been found at Wynniatt, in arctic
Canada ( & ,
1998); although they have not been described in detail, they are suggestive of
Dasycladales and of Ulotrichales. Many of these fossils are multicellular. Very
few specimens have been preserved, which is not surprising given that these
algae, unless they were calcified or silicified, fossilize only in exceptionally
favorable conditions because their wall is most often composed of pectin or
cellulose which decay rapidly.
When
asserts that the oldest known fossils of green algae date back to 750 Ma, he is
presumably thinking of the multicellular green algae that belong to the two
clades of the Chlorophyta that nowadays are the most abundant and diverse, i.e.
the Chlorophyceae and the Ulvophyceae. For an unknown reason he does not
consider the "Prasinophyceae", although they are indisputably
unicellular green algae. Chlorophyceae and Ulvophyceae stand at the top of the
phylogenetic tree of Chlorophyta. They may be called "Neochlorophyta" (see , 2006,
annex 2), meaning that they are preceded by a very long history. Several sites
dated between circa 640 Ma (start of the Varanger glaciation) and circa 770 Ma
have yielded fossils attributed to Dasycladales (Biskopås in Norway: , 1990),
to Ulotrichales (Skillogale in Australia: , 1977),
or more indisputably to Siphonocladales and Sphaeropleales (Svanbergfjellet in
Spitzbergen: et alii, 1994).
Older specimens, dated about 850 Ma, have been found at Wynniatt, in arctic
Canada ( & ,
1998); although they have not been described in detail, they are suggestive of
Dasycladales and of Ulotrichales. Many of these fossils are multicellular. Very
few specimens have been preserved, which is not surprising given that these
algae, unless they were calcified or silicified, fossilize only in exceptionally
favorable conditions because their wall is most often composed of pectin or
cellulose which decay rapidly.
![]() Most of the
oldest specimens that some paleontologists referred to as multicellular green
algae have probably been attributed erroneously (see , 2006,
annex 2). Let's put these questionable cases aside and use established facts in
an attempt to set the record straight. Rather than: "The oldest fossils of
green algae date back 750 Ma", we shall say more accurately: "Between 650 and 850 Ma the green algae were represented by several types of
fossils, some of which belong to evolved clades of Ulvophyceae and
Chlorophyceae. Their presence demonstrates that the radiation of the
multicellular green algae started long before 750 Ma, and that the radiation of
the unicellular green algae is much older" (see , 2006,
annex 3 and
table I
Most of the
oldest specimens that some paleontologists referred to as multicellular green
algae have probably been attributed erroneously (see , 2006,
annex 2). Let's put these questionable cases aside and use established facts in
an attempt to set the record straight. Rather than: "The oldest fossils of
green algae date back 750 Ma", we shall say more accurately: "Between 650 and 850 Ma the green algae were represented by several types of
fossils, some of which belong to evolved clades of Ulvophyceae and
Chlorophyceae. Their presence demonstrates that the radiation of the
multicellular green algae started long before 750 Ma, and that the radiation of
the unicellular green algae is much older" (see , 2006,
annex 3 and
table I ![]() ,
table II
,
table II ![]() ,
table IV
,
table IV ![]() &
table V
&
table V ![]() ).
).
![]() 2. The
Chlorophyta that predominate nowadays (Chlorophyceae, Ulvophyceae and
Trebouxiophyceae) were preceded by the unicellular green algae called
"Prasinophyceae". Molecular analysis of SSU rRNA ( et alii, 1998; et alii,
2000; et alii,
2004; et alii,
2006) revealed that this group is paraphyletic (see , 2006,
annex 1 and table V
2. The
Chlorophyta that predominate nowadays (Chlorophyceae, Ulvophyceae and
Trebouxiophyceae) were preceded by the unicellular green algae called
"Prasinophyceae". Molecular analysis of SSU rRNA ( et alii, 1998; et alii,
2000; et alii,
2004; et alii,
2006) revealed that this group is paraphyletic (see , 2006,
annex 1 and table V ![]() ).
It is made up of six clades (or seven clades, if one separates Nephroselmis
from Pseudoscourfieldia). These unicellular clades diverged successively
from the trunk of the Chlorophyta after the Streptophyta breakoff. The second of
these clades (by seniority) is that of the Pyramimonadales. Several of their
recent representatives possess a distinctive feature that has been highly
favorable to the preservation of their fossil parents: their cycle of life is
not limited to a motile stage during which the alga swims actively using its
flagella; it also comprises a stage that has no exact equivalent in any other
clade, called "phycoma" (see , 2006,
annex 5). Although it is not motile, a phycoma differs from a cyst or a spore in
that it is not inert or "quiescent". Instead the cell remains
metabolically active and its volume increases considerably inside a porous
envelope through which it feeds from the external medium by osmosis. This
envelope, although flexible, is extremely resistant because it is made of a
biopolymer formerly considered to be a sporopollinin but that in some cases is
closer to algaenanes (see , 2006,
annex 14). Pyramimonadales are without contest unicellular green algae, and
their phycomas were fossilized long before 750 Ma, the date
assigns the oldest fossils of green algae. How long before? Here is an attempt
to make such a determination.
).
It is made up of six clades (or seven clades, if one separates Nephroselmis
from Pseudoscourfieldia). These unicellular clades diverged successively
from the trunk of the Chlorophyta after the Streptophyta breakoff. The second of
these clades (by seniority) is that of the Pyramimonadales. Several of their
recent representatives possess a distinctive feature that has been highly
favorable to the preservation of their fossil parents: their cycle of life is
not limited to a motile stage during which the alga swims actively using its
flagella; it also comprises a stage that has no exact equivalent in any other
clade, called "phycoma" (see , 2006,
annex 5). Although it is not motile, a phycoma differs from a cyst or a spore in
that it is not inert or "quiescent". Instead the cell remains
metabolically active and its volume increases considerably inside a porous
envelope through which it feeds from the external medium by osmosis. This
envelope, although flexible, is extremely resistant because it is made of a
biopolymer formerly considered to be a sporopollinin but that in some cases is
closer to algaenanes (see , 2006,
annex 14). Pyramimonadales are without contest unicellular green algae, and
their phycomas were fossilized long before 750 Ma, the date
assigns the oldest fossils of green algae. How long before? Here is an attempt
to make such a determination.
![]() 3.
(1962) noticed that the definition of the fossil genus Tasmanites by
(1958) applies exactly to the recent Pyramimonadale described by
(1899) as Pachysphaera. Among the many species of the genus Tasmanites
(see , 2006,
annex 6), at least two go back to "Precambrian". One of these, T. vindhyanensis,
comes from the Suket Shale at Rampura, India, the date of which is
controversial. The second, T. rifejicus, was found first at sites dated
between 800 and 950 Ma, like Vadsø (Norway), Veteranen (Spitzbergen), Podinzer
(Russia), the Red Pine Shale of the Uinta Mounts and the Galeros Formation of
the Chuar Group (USA). It has been described recently from older sites: et alii
(1999) found it in the Thule Basin of Greenland, circa 1200 Ma. The diameters
(63 - 77 µm) of the Thule Tasmanites are intermediate between those of
the two species described by (1983) from
the Silurian of Missouri. The only difference is that the pores of the wall are
usually slightly smaller (diameter 0.5 µm rather than 1.0 µm).
3.
(1962) noticed that the definition of the fossil genus Tasmanites by
(1958) applies exactly to the recent Pyramimonadale described by
(1899) as Pachysphaera. Among the many species of the genus Tasmanites
(see , 2006,
annex 6), at least two go back to "Precambrian". One of these, T. vindhyanensis,
comes from the Suket Shale at Rampura, India, the date of which is
controversial. The second, T. rifejicus, was found first at sites dated
between 800 and 950 Ma, like Vadsø (Norway), Veteranen (Spitzbergen), Podinzer
(Russia), the Red Pine Shale of the Uinta Mounts and the Galeros Formation of
the Chuar Group (USA). It has been described recently from older sites: et alii
(1999) found it in the Thule Basin of Greenland, circa 1200 Ma. The diameters
(63 - 77 µm) of the Thule Tasmanites are intermediate between those of
the two species described by (1983) from
the Silurian of Missouri. The only difference is that the pores of the wall are
usually slightly smaller (diameter 0.5 µm rather than 1.0 µm).
![]() Are there
older ones? Maybe. Structures described as coarse, but true pores, not as the
result of degradation ( & ,
1992), have been reported on some specimens of the acritarch Trematosphaeridium holtedahlii
at Zigazino-Komarovsk, circa 1350 Ma, and at Bakal, circa 1500 Ma (,
1982). Are these coarse pores forerunners of the smaller and more regularly
disposed pores of Tasmanites? We cannot
affirm this. However, fossils of the green alga Tasmanites certainly
date back to 1200 Ma (not just 750 Ma) and it is possible, although not
demonstrated, that they had forerunners circa 1350 Ma, perhaps even 1500 Ma ago.
Are there
older ones? Maybe. Structures described as coarse, but true pores, not as the
result of degradation ( & ,
1992), have been reported on some specimens of the acritarch Trematosphaeridium holtedahlii
at Zigazino-Komarovsk, circa 1350 Ma, and at Bakal, circa 1500 Ma (,
1982). Are these coarse pores forerunners of the smaller and more regularly
disposed pores of Tasmanites? We cannot
affirm this. However, fossils of the green alga Tasmanites certainly
date back to 1200 Ma (not just 750 Ma) and it is possible, although not
demonstrated, that they had forerunners circa 1350 Ma, perhaps even 1500 Ma ago.
![]() 4. Pterosperma
is, like Pachysphaera, a Pyramimonadale present in existing plankton. Its
phycoma displays a rather unusual feature. The thick, often porous wall produces
one or several wing-shaped expansions perpendicular to its surface (these
expansions are the motive for its name, meaning in Greek "winged
spore"). When there is a single expansion, it has the shape of a large ring
around the equator of the phycoma. This ring characterizes Paleozoic
Pterospermellaceae. The Silurian Pterospermella scruposa left
magnificent examples of these (, 1983). The
genus Pterospermella was common in the earliest Cambrian. Before that time (see
references in , 2006,
annex 7) it occurs at Muhos (Finland, ca. 650 Ma), at Podinzer
(Russia, ca. 900 Ma) and again at Thule (Greenland, ca. 1200 Ma). Pterospermopsimorpha
preceded it, but this name was used as a general waste-basket for many
Precambrian acritarchs of which the so-called wings are actually degraded
protoplasmic residues. However, several specimens from Russia are to all
appearances genuine "pteromorphs": P. insolita at Il'yushkana
(ca. 1200), P. capsulata at Zigazino-Komarovsk (ca. 1350). Eomarginata striata,
at Bakal (ca. 1500 Ma) and at Satka (ca. 1550 Ma), is even older (for references
concerning these very old species see & ,
1992).
Consequently, unless one would assert that no Precambrian pteromorph is related
to the existing Pterosperma, and that resemblances are all convergencies,
this lineage of the green algae, like that of Tasmanites, dates back
indubitably to 1200 Ma, probably to 1350 Ma and perhaps to 1500-1550 Ma (cf.
Table V
4. Pterosperma
is, like Pachysphaera, a Pyramimonadale present in existing plankton. Its
phycoma displays a rather unusual feature. The thick, often porous wall produces
one or several wing-shaped expansions perpendicular to its surface (these
expansions are the motive for its name, meaning in Greek "winged
spore"). When there is a single expansion, it has the shape of a large ring
around the equator of the phycoma. This ring characterizes Paleozoic
Pterospermellaceae. The Silurian Pterospermella scruposa left
magnificent examples of these (, 1983). The
genus Pterospermella was common in the earliest Cambrian. Before that time (see
references in , 2006,
annex 7) it occurs at Muhos (Finland, ca. 650 Ma), at Podinzer
(Russia, ca. 900 Ma) and again at Thule (Greenland, ca. 1200 Ma). Pterospermopsimorpha
preceded it, but this name was used as a general waste-basket for many
Precambrian acritarchs of which the so-called wings are actually degraded
protoplasmic residues. However, several specimens from Russia are to all
appearances genuine "pteromorphs": P. insolita at Il'yushkana
(ca. 1200), P. capsulata at Zigazino-Komarovsk (ca. 1350). Eomarginata striata,
at Bakal (ca. 1500 Ma) and at Satka (ca. 1550 Ma), is even older (for references
concerning these very old species see & ,
1992).
Consequently, unless one would assert that no Precambrian pteromorph is related
to the existing Pterosperma, and that resemblances are all convergencies,
this lineage of the green algae, like that of Tasmanites, dates back
indubitably to 1200 Ma, probably to 1350 Ma and perhaps to 1500-1550 Ma (cf.
Table V ![]() ).
).
![]() 5. Some of the
species assigned to the existing genus Pterosperma bear, instead of a
single equatorial ring, several membranous expansions, crests or
"wings" that give to the external surface of the phycoma a reticulate
appearance. This particularity is also found in fossil Cymatiosphaeraceae (,
1963). Many genera classified as in this "family" have been designated
as "herkomorph acritarchs" and are characterized by a surface divided
into polygonal fields by crests. In earliest Cambrian times Cymatiosphaera
is represented by eight species, that appear to have been preceded by C. precambrica
at Hailuoto, Finland, ca. 650 Ma, and by C. sp. at Kandyk, Siberia, ca. 700 Ma
(see & ,
1992). The forerunner of this genus, ca. 775 Ma, is a fossil from Hunnberg,
Greenland, and from Visingsö, Sweden, named by
(1976) Peteinosphaeridium
reticulatum. Today this species has been referred to the genus
Vandalosphaeridium - a genus that had its own forerunner at Thule ca. 1200 Ma ( et alii,
1999). Nothing is known to suggest that the history of
the herkomorphs begins earlier. It is difficult to assume that an enigmatic
acritarch of the Belt Supergroup in Montana, ca. 1450 Ma (,
1980), has
more in common with them than a fortuitous resemblance.
5. Some of the
species assigned to the existing genus Pterosperma bear, instead of a
single equatorial ring, several membranous expansions, crests or
"wings" that give to the external surface of the phycoma a reticulate
appearance. This particularity is also found in fossil Cymatiosphaeraceae (,
1963). Many genera classified as in this "family" have been designated
as "herkomorph acritarchs" and are characterized by a surface divided
into polygonal fields by crests. In earliest Cambrian times Cymatiosphaera
is represented by eight species, that appear to have been preceded by C. precambrica
at Hailuoto, Finland, ca. 650 Ma, and by C. sp. at Kandyk, Siberia, ca. 700 Ma
(see & ,
1992). The forerunner of this genus, ca. 775 Ma, is a fossil from Hunnberg,
Greenland, and from Visingsö, Sweden, named by
(1976) Peteinosphaeridium
reticulatum. Today this species has been referred to the genus
Vandalosphaeridium - a genus that had its own forerunner at Thule ca. 1200 Ma ( et alii,
1999). Nothing is known to suggest that the history of
the herkomorphs begins earlier. It is difficult to assume that an enigmatic
acritarch of the Belt Supergroup in Montana, ca. 1450 Ma (,
1980), has
more in common with them than a fortuitous resemblance.
![]() Several
morphotypes of acritarchs may have belonged to now extinct lineages of
Pyramimonadales: the "polygonomorph" Podolina at Båtsfjord
ca. 730 Ma, the "prismatomorphs" Octoexydrium at Lakhanda ca.
1030 Ma and Quadratimorpha at Hongshuizhuang ca. 1250 Ma and Wumishan
ca. 1320 Ma (see & ,
1992). Simia annulare, characterized by its double envelope, lieved at
Thule in sympatry with the closely related Pterospermella ca. 1200 Ma ( et alii,
1999).
Several
morphotypes of acritarchs may have belonged to now extinct lineages of
Pyramimonadales: the "polygonomorph" Podolina at Båtsfjord
ca. 730 Ma, the "prismatomorphs" Octoexydrium at Lakhanda ca.
1030 Ma and Quadratimorpha at Hongshuizhuang ca. 1250 Ma and Wumishan
ca. 1320 Ma (see & ,
1992). Simia annulare, characterized by its double envelope, lieved at
Thule in sympatry with the closely related Pterospermella ca. 1200 Ma ( et alii,
1999).
![]() The
acanthomorphs, or "thorny acritarchs", are clearly a polyphyletic
collection. Histricosphaera, the "porcupine ball" that lent its
name to the "class Hystrichophyta" (,
1963), is actually a Dinoflagellate. The presence of the biomarker dinosteran
indicates that four genera from the lower Cambrian of Lükati, Estonia, Comasphaeridium, Lophosphaeridum, Globosphaeridium
and Skagia, are Dinoflagellates too ( et alii,
2000). However other acantomorphs may well be Pyramimonadales. A spiny
herkomorph such as Dictyotidium from Svanbergfjellet ( et alii, 1994)
is transitional in the same way as the acanthomorph Vandalosphaeridium and
its forerunner from Thule. It is therefore very possible that lineages of
acanthomorphic Pyramimonadales, now extinct, coexisted in Greenland about 1200 Ma
with three lineages of spheromorphic Pyramimonadales, namely Tasmanites, Pterospermella
and Simia.
The
acanthomorphs, or "thorny acritarchs", are clearly a polyphyletic
collection. Histricosphaera, the "porcupine ball" that lent its
name to the "class Hystrichophyta" (,
1963), is actually a Dinoflagellate. The presence of the biomarker dinosteran
indicates that four genera from the lower Cambrian of Lükati, Estonia, Comasphaeridium, Lophosphaeridum, Globosphaeridium
and Skagia, are Dinoflagellates too ( et alii,
2000). However other acantomorphs may well be Pyramimonadales. A spiny
herkomorph such as Dictyotidium from Svanbergfjellet ( et alii, 1994)
is transitional in the same way as the acanthomorph Vandalosphaeridium and
its forerunner from Thule. It is therefore very possible that lineages of
acanthomorphic Pyramimonadales, now extinct, coexisted in Greenland about 1200 Ma
with three lineages of spheromorphic Pyramimonadales, namely Tasmanites, Pterospermella
and Simia.
![]() 6. Certainly a
very different type of green algae was present at Ruyang, China, aroud 1200 Ma
ago. Spiromorpha segmentata appears to be very close to the present-day Spirotaenia ( et alii,
2005). It belongs to the Zygnematophyceae
(see , 2006,
annex 12 and
Table III
6. Certainly a
very different type of green algae was present at Ruyang, China, aroud 1200 Ma
ago. Spiromorpha segmentata appears to be very close to the present-day Spirotaenia ( et alii,
2005). It belongs to the Zygnematophyceae
(see , 2006,
annex 12 and
Table III ![]() ), a very derived clade of the Streptophyta. This clade is
characterized by an uncommon mode of sexual reproduction, in which two cells or
two filaments unite through a connecting tube (another name for this clade,
"Conjugaphyceae", remarks on this particularity). The presence of Spiromorpha at Ruyang confirms that some fossil green algae are much older than
750 Ma, and demonstrates that the ancestor common to Streptophyta and
Chlorophyta must have existed long before 1200 Ma.
), a very derived clade of the Streptophyta. This clade is
characterized by an uncommon mode of sexual reproduction, in which two cells or
two filaments unite through a connecting tube (another name for this clade,
"Conjugaphyceae", remarks on this particularity). The presence of Spiromorpha at Ruyang confirms that some fossil green algae are much older than
750 Ma, and demonstrates that the ancestor common to Streptophyta and
Chlorophyta must have existed long before 1200 Ma.
![]() 7. In 1899
described, along with Pachysphaera, another Pyramimonadale from the same
planktonic assemblage, Halosphaera. This genus differs from Pachysphaera
in its lesser diameter, its much thinner envelope and by the absence of pores.
(1962) wanted to complete the symmetry: just as he considered that the fossil Tasmanites
was related to Pachysphaera, he postulated the same relationship between Halosphaera
and a fossil exceedingly abundant and extremely old, Leiosphaeridia. In
the Thule Basin, ca. 1200 Ma, tens of thousands of specimens have been counted ( et alii,
1999). Those from Chuanlinggou, in China, date back to ca. 1730 Ma ( et alii,
2003).
7. In 1899
described, along with Pachysphaera, another Pyramimonadale from the same
planktonic assemblage, Halosphaera. This genus differs from Pachysphaera
in its lesser diameter, its much thinner envelope and by the absence of pores.
(1962) wanted to complete the symmetry: just as he considered that the fossil Tasmanites
was related to Pachysphaera, he postulated the same relationship between Halosphaera
and a fossil exceedingly abundant and extremely old, Leiosphaeridia. In
the Thule Basin, ca. 1200 Ma, tens of thousands of specimens have been counted ( et alii,
1999). Those from Chuanlinggou, in China, date back to ca. 1730 Ma ( et alii,
2003).
![]() Immediately a
difficulty arose. Where there is no thorn, no pore and no carved ornament, just
a uniformly smooth sphere, how is it possible to determine whether or not a Leiosphaeridia
is really the phycoma of a Pyramimonadale ? Morphology is not enough. It is
necessary to use more precise methods, to analyze the ultrastructure and the
biochemical components of the envelopes, to compare the ways in which dehiscence
takes place (see ,
2006, annexes 9-10 and 14). When this has been
done, one is faced with the compelling evidence that the name Leiosphaeridia
has been applied to an extremely heterogenous assemblage (see ,
2006, annex 11). Most of these fossils have no
affinity whatsoever with Halosphaera. However some of them are almost
unquestionably Pyramimonadales or more "advanced" Chlorophyta. For
example L. crassa, one of three Leiosphaeridia
"species" from Roper in Australia, ca. 1450 Ma, displays in the outer
layer of its wall a trilaminar structure that is a characteristic of the
Chlorophyceae ( et alii, 2004). Barring
an accidental, remotely possible coincidence, the conclusion is that a lineage
of green algae in existence ca. 1450 Ma ago was already progressing toward the
modern "Neochlorophyta".
Immediately a
difficulty arose. Where there is no thorn, no pore and no carved ornament, just
a uniformly smooth sphere, how is it possible to determine whether or not a Leiosphaeridia
is really the phycoma of a Pyramimonadale ? Morphology is not enough. It is
necessary to use more precise methods, to analyze the ultrastructure and the
biochemical components of the envelopes, to compare the ways in which dehiscence
takes place (see ,
2006, annexes 9-10 and 14). When this has been
done, one is faced with the compelling evidence that the name Leiosphaeridia
has been applied to an extremely heterogenous assemblage (see ,
2006, annex 11). Most of these fossils have no
affinity whatsoever with Halosphaera. However some of them are almost
unquestionably Pyramimonadales or more "advanced" Chlorophyta. For
example L. crassa, one of three Leiosphaeridia
"species" from Roper in Australia, ca. 1450 Ma, displays in the outer
layer of its wall a trilaminar structure that is a characteristic of the
Chlorophyceae ( et alii, 2004). Barring
an accidental, remotely possible coincidence, the conclusion is that a lineage
of green algae in existence ca. 1450 Ma ago was already progressing toward the
modern "Neochlorophyta".
![]() 8. It is
exceptional today for a coccoid Prokaryote to reach a diameter of 60 µm (& , 1992). Many spheroid fossils
from the Chuanlinggou site in China, recently dated to circa 1730 Ma ( et alii,
2003), have a diameter larger than 60 µm: that of Stictosphaeridium implexum,
for instance, may attain 103 µm. These "mesospheromorphs" were most
certainly Eukaryotes. Probably they were algae, as their planktonic habitus
suggests. If so, taking into account their size, age and the resistant envelope
that facilitated their preservation, the conclusion that some were
Pyramimonadales is inescapable. The species Schizofusa sinica (& , 1993) has a median exkystment
fissure. No Prokaryote has one, but it is appropriate to a cyst or a phycoma. Tyrasotaenia,
a uniserial filamentous alga, was also found at Chuanlinggou. According to
(1994) it is related to the Vendotaeniids, a now extinct clade of the
Rhodophyta. Therefore it is plausible, although not demonstrated, that an
already advanced lineage of the Chlorophyta (mesospheromorphs producing phycoma)
and an already advanced lineage of the Rhodophyta (filamentous Vendotaeniids)
coexisted in China about 1730 Ma. This relationship suggests that the divergence
of red algae and green algae took place long before 1730 Ma, not a little more
than 1200 Ma, as 's diagram proposes (Life on a young planet,
2003, p. 152, fig. 9.5).
8. It is
exceptional today for a coccoid Prokaryote to reach a diameter of 60 µm (& , 1992). Many spheroid fossils
from the Chuanlinggou site in China, recently dated to circa 1730 Ma ( et alii,
2003), have a diameter larger than 60 µm: that of Stictosphaeridium implexum,
for instance, may attain 103 µm. These "mesospheromorphs" were most
certainly Eukaryotes. Probably they were algae, as their planktonic habitus
suggests. If so, taking into account their size, age and the resistant envelope
that facilitated their preservation, the conclusion that some were
Pyramimonadales is inescapable. The species Schizofusa sinica (& , 1993) has a median exkystment
fissure. No Prokaryote has one, but it is appropriate to a cyst or a phycoma. Tyrasotaenia,
a uniserial filamentous alga, was also found at Chuanlinggou. According to
(1994) it is related to the Vendotaeniids, a now extinct clade of the
Rhodophyta. Therefore it is plausible, although not demonstrated, that an
already advanced lineage of the Chlorophyta (mesospheromorphs producing phycoma)
and an already advanced lineage of the Rhodophyta (filamentous Vendotaeniids)
coexisted in China about 1730 Ma. This relationship suggests that the divergence
of red algae and green algae took place long before 1730 Ma, not a little more
than 1200 Ma, as 's diagram proposes (Life on a young planet,
2003, p. 152, fig. 9.5).
![]() 9. When
fossils are smaller than 60 µm, how can Eukaryotes be differentiated from
Prokaryotes? This depends in part on the answer to another question: how was it
possible for these objects to be preserved?
9. When
fossils are smaller than 60 µm, how can Eukaryotes be differentiated from
Prokaryotes? This depends in part on the answer to another question: how was it
possible for these objects to be preserved?
![]() In 1931
perfected at Tübingen University a method for extracting the pollen seeds of
fossil plants from their gangue. He soaked the matrix containing the pollen in
hydrofluoric acid to which the pollen wall is resistant.
(1969) has used this drastic treatment since 1958 in order to extract
acritarchs from the Proterozoic sediments of Siberia and the Ural Mountains.
In 1931
perfected at Tübingen University a method for extracting the pollen seeds of
fossil plants from their gangue. He soaked the matrix containing the pollen in
hydrofluoric acid to which the pollen wall is resistant.
(1969) has used this drastic treatment since 1958 in order to extract
acritarchs from the Proterozoic sediments of Siberia and the Ural Mountains.
![]() Commonly, the
wall of a vegetal cell consists of water soluble polysaccharids, such as
cellulose, that usually are not preserved. However there are exceptions. Early
in the 1990s, these exceptions were all considered to be due to the presence of
a substance called sporopollinin, defined as a family of water insoluble and
acid resistant biopolymers of high molecular weight. Since then it has been
established that several distinct classes of resistant biopolymers exist, the
most well-defined being dinosporin and algaenans. Dinosporin characterizes
Dinoflagellates, so is not of interest here. But in most Chlorophyta the
biopolymers are algaenans. Their composition and definition pose a lot of
problems: not all the macrobiomolecules grouped under this name are homologous;
they have not always been clearly distinguished from sporopollinins; many
resistant biopolymers are neither algaenans nor sporopollinins; last, the long
entombment of the fossil in sedimentary rocks commonly altered to some degree
often causes changes in its chemical composition to the point that the initial
state of its polymers is no longer identifiable (see ,
2006, annexes 14-15). Therefore, instead of using
terms which only seemingly are more precise scientifically, it is prudent to
stick to 's concept (1992) of a cellular
wall prone to fossilization because the biopolymers it contains are resistant to
hydrolysis and acetolysis.
Commonly, the
wall of a vegetal cell consists of water soluble polysaccharids, such as
cellulose, that usually are not preserved. However there are exceptions. Early
in the 1990s, these exceptions were all considered to be due to the presence of
a substance called sporopollinin, defined as a family of water insoluble and
acid resistant biopolymers of high molecular weight. Since then it has been
established that several distinct classes of resistant biopolymers exist, the
most well-defined being dinosporin and algaenans. Dinosporin characterizes
Dinoflagellates, so is not of interest here. But in most Chlorophyta the
biopolymers are algaenans. Their composition and definition pose a lot of
problems: not all the macrobiomolecules grouped under this name are homologous;
they have not always been clearly distinguished from sporopollinins; many
resistant biopolymers are neither algaenans nor sporopollinins; last, the long
entombment of the fossil in sedimentary rocks commonly altered to some degree
often causes changes in its chemical composition to the point that the initial
state of its polymers is no longer identifiable (see ,
2006, annexes 14-15). Therefore, instead of using
terms which only seemingly are more precise scientifically, it is prudent to
stick to 's concept (1992) of a cellular
wall prone to fossilization because the biopolymers it contains are resistant to
hydrolysis and acetolysis.
![]() Left aside the
phycomas of Pyramimonadales, this kind of cellular wall is found in only three
types of spheromorphs:
Left aside the
phycomas of Pyramimonadales, this kind of cellular wall is found in only three
types of spheromorphs:
![]() a) the pollen and other spores used by sexual plants for reproduction, notably
the gyronites of the Charophyceae (Chara, Nitella, Coleochaete);
a) the pollen and other spores used by sexual plants for reproduction, notably
the gyronites of the Charophyceae (Chara, Nitella, Coleochaete);
![]() b) the "quiescent cysts" in which some green algae and Dinoflagellates
enclose themselves when the environment is unfavorable to their growth;
b) the "quiescent cysts" in which some green algae and Dinoflagellates
enclose themselves when the environment is unfavorable to their growth;
![]() c) the envelope of vegetative cells of some unicellular algae, most often
Chlorophyta (Chlorella, Pediastrum, Scenedesmus) but also
some Dinoflagellates and Heterokonta (Eustigmatophyceae). To these must be added
non-spheromorphic fossils like the zygospores of some Charophyta such as
Zygnematophyceae. Biopolymers of unknown types must be listed too: these, because
they have been preserved, must contain the lorica of some Euglenozoa (,
1981), the theca of "Melanocyrilliid" amoebae or the envelopes of Chuaria
and Tawuia.
c) the envelope of vegetative cells of some unicellular algae, most often
Chlorophyta (Chlorella, Pediastrum, Scenedesmus) but also
some Dinoflagellates and Heterokonta (Eustigmatophyceae). To these must be added
non-spheromorphic fossils like the zygospores of some Charophyta such as
Zygnematophyceae. Biopolymers of unknown types must be listed too: these, because
they have been preserved, must contain the lorica of some Euglenozoa (,
1981), the theca of "Melanocyrilliid" amoebae or the envelopes of Chuaria
and Tawuia.
![]() However, with
the exception of the Dinoflagellates, resistance to hydrolysis and acetolysis is
most often a property of Viridiplantae. Some acritarchs have been found in Tyler
(Michigan) ca. 1950 Ma, in Epworth (Canada) ca. 1920 Ma and in Frere (Australia)
ca. 1870 Ma (see references in & ,
1992), but the great
majority of specimens dated between 1800 et 2000 Ma come from Russian sites
where and his followers used 's method for extracting
acritarchs from their matrix. Few other fossils resist this drastic treatment,
and nearly all of them belong to only one type: they are colonial coccoid
Cyanobacteria protected by a collective mucilaginous-like envelope (see
Table VII
However, with
the exception of the Dinoflagellates, resistance to hydrolysis and acetolysis is
most often a property of Viridiplantae. Some acritarchs have been found in Tyler
(Michigan) ca. 1950 Ma, in Epworth (Canada) ca. 1920 Ma and in Frere (Australia)
ca. 1870 Ma (see references in & ,
1992), but the great
majority of specimens dated between 1800 et 2000 Ma come from Russian sites
where and his followers used 's method for extracting
acritarchs from their matrix. Few other fossils resist this drastic treatment,
and nearly all of them belong to only one type: they are colonial coccoid
Cyanobacteria protected by a collective mucilaginous-like envelope (see
Table VII ![]() ).
).
![]() Several clades of "Prasinophyceae" that do not form phycoma
comprise a large part of the present-day nanoplankton and picoplankton. The
extreme intraspecific diversity of the Mamiellales was detected only recently.
At the same time, new clades were discovered: Pycnococcaceae, Picocystidales,
Prasinococcales ( et alii, 2004). Most of these algae are very small,
their diameter being 3 µm or even less: Ostreococcus tauri and Bathycoccus prasinos rarely attain 1 µm. If 's criterion was applied to these living
"microspheromorphs", all of them would be classified as "coccoid
bacteria", none as an "acritarch", although they are indisputably
green algae.
Several clades of "Prasinophyceae" that do not form phycoma
comprise a large part of the present-day nanoplankton and picoplankton. The
extreme intraspecific diversity of the Mamiellales was detected only recently.
At the same time, new clades were discovered: Pycnococcaceae, Picocystidales,
Prasinococcales ( et alii, 2004). Most of these algae are very small,
their diameter being 3 µm or even less: Ostreococcus tauri and Bathycoccus prasinos rarely attain 1 µm. If 's criterion was applied to these living
"microspheromorphs", all of them would be classified as "coccoid
bacteria", none as an "acritarch", although they are indisputably
green algae.
![]() Eukaryotic
phylogenetic trees suggest that most acritarchs dated between 1800 and 2000 Ma
must be either Chlorophyta even older than the Pyramimonadales (only one such
clade has still representatives today, the Prasinococcales), or extremely
archaic Charophyta like the Chlorokybales and Mesostigmatales. If these ancient
acritarchs are none of these (and this is becoming more and more probable with
the approach to 2000 Ma), they may represent the stem-group of the
Viridiplantae, i.e. the common ancestor of the Chlorophyta and the
Streptophyta.
Eukaryotic
phylogenetic trees suggest that most acritarchs dated between 1800 and 2000 Ma
must be either Chlorophyta even older than the Pyramimonadales (only one such
clade has still representatives today, the Prasinococcales), or extremely
archaic Charophyta like the Chlorokybales and Mesostigmatales. If these ancient
acritarchs are none of these (and this is becoming more and more probable with
the approach to 2000 Ma), they may represent the stem-group of the
Viridiplantae, i.e. the common ancestor of the Chlorophyta and the
Streptophyta.
![]() Many
discoveries made in the last ten years in the field of Precambrian paleontology
suggest that Eukaryote radiation can be traced farther back in time than was
thought previously. Only five milestones among the more significant ones are
outlined here:
Many
discoveries made in the last ten years in the field of Precambrian paleontology
suggest that Eukaryote radiation can be traced farther back in time than was
thought previously. Only five milestones among the more significant ones are
outlined here:
- A highly complex assembly of Eukaryotes already existed circa 1100-1200 Ma in the Thule Supergroup of Greenland ( et alii, 1999). It includes at least three discrete types of Pyramimonadales, along with various spheromorph and acanthomorph acritarchs.
- The Eukaryotes had already reached a high level of diversity in the Roper Group, Australia, circa 1450 Ma ( et alii, 2001, 2004).
- Multicellular organisms exhibiting a functional differentiation between several cell types, the Longfengshaniids, occur in the Tuanshanzi Formation, China, circa 1650-1700 Ma ( & , 1995).
- The date of the sudden increase in atmospheric oxygen at the beginning of the Proterozoic has been determined precisely. It occurred between the two last Huronian glaciations, i.e. between 2450 and 2320 Ma ( et alii, 2004).
- The presence of Eukaryotes circa 2700 Ma at Wittenoom, Australia, is attested by sterans, biomarkers that only Eukaryotes can synthesize ( et alii, 1999).
Note. A discovery requires confirmation. In the Dashiling and Qingshicun Formations of the Hutuo Group, China, ca. 2400 Ma, & (1998) have collected microfossils that they have assignated to 19 genera and 31 species. Among them are large spheromorphs, coccoids connected by a filament (Polysphaeroides formosus) and an enigmatic triangular theca (Triangulomorpha crassa) that have been interpreted as Eukaryotes. If this attribution is correct, and if these fossils are actually 2400 millions years old, Precambrian chronology will have to be reconsidered.
![]() All these
discoveries are posterior to 's famous paper on
the "big bang" of the Eukaryotic crown-group (1992).
They render less plausible the hypothesis that he was defending then, and that
is still the basis for diagram 9.5 in his book, Life on a young planet
(, 2003, p. 152, fig. 9.5).
They are more in agreement, I think, with several ideas that I defended in my
book, La vie invisible (, 2002),
and that I attempt to delineate in this paper:
All these
discoveries are posterior to 's famous paper on
the "big bang" of the Eukaryotic crown-group (1992).
They render less plausible the hypothesis that he was defending then, and that
is still the basis for diagram 9.5 in his book, Life on a young planet
(, 2003, p. 152, fig. 9.5).
They are more in agreement, I think, with several ideas that I defended in my
book, La vie invisible (, 2002),
and that I attempt to delineate in this paper:
- At about 750 Ma the evolution of multicellular green algae was already far advanced.
- Several specialized types of Pyramimonadales, of which two (Pachysphaera and Pterosperma) still exist today, were present more than 1200 Ma ago. Some of them may go back as far as 1500 Ma.
- The presence in the 1150-1250 Ma Ruyang Group of a very derived type of Streptophyta, the Zygnematale Spiromorpha, indisputably implies a long prior evolution of Viridiplantae.
- Phycomas of Pyramimonadales may be as old as 1730 Ma. In any case, acritarchs similar in their "mesospheromorphic" size and their mode of dehiscence to present-day phycomas existed at Chuanlinggou at this time.
- Small acritarchs, the envelope of which contained an acetolysis-resistant biopolymer, were numerous and diverse from 2000 Ma. Probably among them were primitive Viridiplantae.
![]() The date of
750 Ma, from which we started, is far behind us.
The date of
750 Ma, from which we started, is far behind us.
![]() The author thanks Emmanuelle , Alain and an anonymous reviewer for having led
him, by their constructive suggestions, to improve a first version of the manuscript. Special thanks are due to
Claudine for having provided an English translation of this first
version and to Nestor for his assistance in
amending the final English text.
The author thanks Emmanuelle , Alain and an anonymous reviewer for having led
him, by their constructive suggestions, to improve a first version of the manuscript. Special thanks are due to
Claudine for having provided an English translation of this first
version and to Nestor for his assistance in
amending the final English text.
K.R., P.F. & M.R. (1999).- A possible chlorophycean affinity of some Neoproterozoic acritarchs.- Organic Geochemistry, Amsterdam, vol. 30, n° 10, p. 1323-1337.
K.R., P.F. & M.R. (2000).- Biological affinities of Neoproterozoic acritarchs from Australia: microscopic and chemical characterisation - the Savory and Officer Basin.- Organic Geochemistry, Amsterdam, vol. 31, n° 1, p. 75-89.
A., H.D., P.-L., D. III, H.J., J.L., L.L. & N.J. (2004).- Dating the rise of atmospheric oxygen.- Nature, London, vol. 427, n° 6970, p. 117-120.
A. & D. (1999).- Evolutionary analyses of small-subunit rDNA coding regions and the 1506 group I introns of Zygnematales (Charophyceae, Streptophyta).- Journal of Phycology, Oxford, vol. 35, n° 3, p. 560-569.
G.C., G.L. & P.A. (1998a).- Polyphyly of Tetrasporalean green algae inferred from nuclear small-subunit ribosomal DNA.- Journal of Phycology, Oxford, vol. 34, n° 2, p. 306-311.
G.C., G.L. & P.A. (1998b).- Origins and affinities of the filamentous green algal orders Chaetophorales and Oedogoniales based on 18S rRNA gene sequences.- Journal of Phycology, Oxford, vol. 34, n° 2, p. 312-318.
J.J., G.A., R. & R.E. (1999).- Archaean molecular fossils and the early rise of Eukaryotes.- Science, Washington, vol. 285, n° 5430, p. 1033-1036.
M.A., E.A. & J.A. (2001).- Phylogeny of the Chlorophyceae with special reference to the Sphaeropleales: a study of 18S and 26S rDNA data.- Journal of Phycology, Oxford, vol. 37, n° 5, p. 819-835.
N.J., A.H. & K. (1994).- Paleobiology of the Neoproterozoic Svanbergfjellet Formation, Spitsbergen.- Fossils and Strata, Oslo, n° 34, 84 p.
N.J. & R.H. (1998).- Diverse organic-walled fossils, including "possible dinoflagellates", from the early Neoproterozoic of arctic Canada.- Geology, Boulder, vol. 26, n° 11, p. 963-966.
T. (1981).- Eukaryote kingdoms: seven or nine?.- BioSystems, Amsterdam, vol. 14, n° 3-4, p. 461–481.
G.K. (1983).- Fossil prasinophycean phycomata (Chlorophyta) from the Silurian Bainbridge Formation, Missouri, U.S.A.- Phycologia, Lawrence, vol. 22, n° 3, p. 249-265.
T., D. & T. (2001).- Monophyly of the genus Closterium and the order Desmidiales (Charophyceae, Chlorophyta) inferred from nuclear small subunit rDNA data.- Journal of Phycology, Oxford, vol. 37, n° 6, p. 1063-1072.
C.S., J., K.G., C.F. & R.M. (2005).- Phylogeny of Spirogyra and Sirogonium (Zygnematophyceae) based on rbcL sequence data.- Journal of Phycology, Oxford, vol. 41, n° 5, p. 1055-1064.
A. (1931).- Neue Mikrofossilien des baltischen Silurs. 1.- Palaeontologische Zeitschrift, Stuttgart, vol. 13, n° 1-2, p. 74-118.
A. (1958).- Tasmanites 1875 und Leiosphaeridia n.g. als Gattungen der Hystrichosphaerida.- Palaeontographica. Abteilung A, Stuttgart, vol. 110, n° 1-3, p. 1-24.
M.W., Y. & M. (2000).- Phylogenetic analyses of 18S rDNA sequences reveal a new coccoid lineage of the Prasinophyceae (Chlorophyta).- Journal of Phycology, Oxford, vol. 36, n° 2, p. 387-393.
T. (1997).- The evolution of green algae.- In : D. (ed.), The origin of algae and plastids.- Springer Verlag, Wien, p. 87-101.
A.A. & M. (2005).- Molecular phylogeny of Staurastrum ex and related genera (Zygnematophyceae, Streptophyta) based on coding and noncoding rDNA sequence comparisons.- Journal of Phycology, Oxford, vol. 41, n° 4, p. 887-899.
L., W., M-J., F., R., K., C. & D. (2004).- Diversity of picoplanktonic prasinophytes assessed by direct nuclear SSU rDNA sequencing of environmental samples and novel isolates retrieved from oceanic and coastal marine ecocystems.- Protist, Jena, vol. 155, n° 2, p. 193-214.
D., E. & L. (2000).- Phylogenetic position of the Oocystaceae (Chlorophyta).- Journal of Phycology, Oxford, vol. 36, n° 3, p. 590-595.
H.J. (1994).- Proterozoic carbonaceous compressions ("metaphytes" and "worms").- In: S. (ed.), Early life on Earth. Nobel Symposium n° 84.- Columbia University Press, New York, p. 342-357.
R.J. (1980).- Middle Proterozoic shale-facies microbiota from the lower Belt Supergroup, Little Belt Mountains, Montana.- Journal of Paleontology, Tulsa, vol. 54, n° 4, p. 649-663.
R.J., J., J.H. & C.V. (1992).- Preservation of prokaryotes and organic-walled and calcareous and siliceous protists.- In: J.W. & C. (eds.), The Proterozoic biosphere. A multidisciplinary study.- Cambridge University Press, p. 185-193.
E.J., A.H. & M.R. (2001).- Morphological and ecological complexity in early eukaryotic ecosystems.- Nature, London, vol. 412, n° 6842, p. 66-69.
E.J., A.H. & M.R. (2004).- TEM evidence for eukaryotic diversity in mid-Proterozoic oceans.- Geobiology, Oxford, vol. 2, n° 3, p. 121-132.
K.G., R.M., M.T. & C.F. (2001).- The closest living relatives of land plants.- Science, Washington, vol. 294, n° 5550, p. 2351-2353.
A.H. (1992).- The early evolution of eukaryotes – a geological perspective.- Science, Washington, vol. 256, n° 5057, p. 622-627.
A.H. (2003).- Life on a young planet. The first three billion years of evolution on Earth.- Princeton University Press, third edition, 277 p.
L., I., T. & V.A.R. (2001).- Traditional generic concepts versus 18 rRNA phylogeny in the green algal family Selenestraceae (Chlorophyceae, Chlorophyta).- Journal of Phycology, Oxford, vol. 37, n° 5, p. 852-867.
C., C. & M. (2000).- Ancestral chloroplast genome in Mesostigma viride reveals an early branch of green plant evolution.- Nature, London, vol. 403, p. 649-652.
C., P., G., J. & Y. (2003).- A molecular and isotopic geochemical study of Meso- to Neoproterozoic (1.73-0.85 Ga) sediments from the Jixian section, Yanshan Basin, North China.- Precambrian Research, Amsterdam, vol. 125, n° 3, p. 337-356.
S. (1981).- Remarks on the taxinomy, botanical affinities, and distribution of leiospheres.- Stockholm Contributions in Geolology, vol. 38, n° 1, p. 1-20.
R.M., K.G., J., K.M., A., M.F. & R.W. (2000).- Phylogeny of the conjugating green algae (Zygnemophyceae) based on rbcL sequences.- Journal of Phycology, Oxford, vol. 36, n° 4, p. 747-758.
K. (1963).- Charophyten und Halophyten.- Fortschritte der Geologie in Rheinland und Westfalen, Krefeld, Band 10, p. 121-128.
C.P., E.J., A.H. & M.R. (2005).- Combined micro-Fourier transform infrared (FTIR) spectroscopy and micro-Raman spectroscopy of Proterozoic acritarchs: A new approach to Palaeobiology.- Precambrian Research, Amsterdam, vol. 138, n° 3-4, p. 208-224.
C.V. & J.W. (1992).- Proterozoic and selected Early Cambrian microfossils and microfossil-like objects.- In: J.W. & C. (eds.), The Proterozoic biosphere. A multidisciplinary study.- Cambridge University Press, p. 867-951.
T., B., H.D., B., V.A.R., I. & M. (1998).- The basal position of scaly green flagellates among the green algae (Chlorophyta) is revealed by analyses of nuclear-encoded SSU rRNA sequences.- Protist, Jena, vol. 149, n° 4, p. 367-380.
C.H. (1899).- Plankton.- In: C.F., M. & C.H. (eds.), Iagttagelser over overfladevandets temperatur, saltholdighed og plankton paa islandske og grønlandske skibsrouter.– Bianco Lunos Kgl. Hof-Bogtrykkeri, Copenhagen, p. 59. (In Danish).
J.-F., C., C. & M. (2004).- The complete mitochondrial DNA sequence of the green alga Pseudendoclonium akinetum (Ulvophyceae) highlights distinctive evolutionary trends in the chlorophyta and suggests a sister-group relationship between the Ulvophyceae and Chlorophyceae.- Molecular Biology and Evolution, Oxford, vol. 21, n° 5, p. 922-935.
T., B., U.G. & M. (2001).- Molecular phylogeny and taxonomic revision of Chlamydomonas (Chlorophyta). I. Emendation of Chlamydomonas and Chloromonas , and description of Oogamochlamys gen. nov. and Lobochlamys gen. nov.- Protist, Jena, vol. 152, n° 4, p. 265-300.
J., P.R. & G. (1999).- Organic-walled microfossils from Proterozoic Thule Supergroup, Northwest Greenland.- Precambrian Research, Amsterdam, vol. 96, n° 1, p. 1-23.
J.W. (1977).- Biostratigraphic usefulness of stromatolitic Precambrian microbiotas: A preliminary analysis.- Precambrian Research, Amsterdam, vol. 5, p. 143–173.
J.W. & C. (eds.) (1992).- The Proterozoic biosphere. A multidisciplinary study.- Cambridge University Press, 1348 p.
J., P. & D. (2006).- Global dispersal and ancient cryptic species in the smallest marine Eukaryotes.- Molecular Biology and Evolution, Oxford, vol. 23, n° 1, p. 23-29.
R.C. (1955).- A comparative study of Chlorococcum and other spherical, zoospore-producing genera of the Chlorococcales.- Indiana University Science Series, Bloomington, n° 20, 111 p.
S.F. & S.X. (1998).- Discovery of micropaleophytes from the Doucun subgroup (about 2400 Ma), Hutuo Group of Wutai Mountain.- Acta Micropaleontologica Sinica, Nanjing, vol. 15, n° 3, p. 286-293.
N.M., J.M., A. & F.J. (2000).- Affinities of Early Cambrian acritarchs studied by using microscopy, fluorescence flow cytometry and biomarkers.- Review of Palaeobotany and Palynology, Amsterdam, vol. 108, n° 1-2, p. 37-53.
N.M. & M. (2000).- Morphological and ultrastructural studies of some acritarchs from the Lower Cambrian Lükati Formation, Estonia.- Review of Palaeobotany and Palynology, Amsterdam, vol. 112, n° 1-3, p. 1-21.
B. (2002).- La vie invisible. Les trois premiers milliards d'années de l'histoire de la vie sur terre.- L'Harmattan, Paris, 482 p.
B. (2006).- Les algues vertes (phylum Viridiplantae), sont-elles vieilles de deux milliards d'années ?.- Carnets de Géologie, Brest, Book 2006/01 (CG2006_B01)
B.V. (1966).- Micropaleofitologicheskoe Issledovanie Drevnikh Svit [Microphytological investigations of ancient formations].- Akademiya Nauk SSSR, Laboratoriya Geologii Dokembriya, Izdatel'stvo "Nauka", Moscow, 147 p. + LXXXIX pl. (in Russian)
B.V. (1969).- Sferomorfidy Proterozoya [Proterozoic Sphaeromorphida].- Akademiya Nauk SSSR, Institut Geologii i Geokhronologii Dokembriya, Izdatel'stvo "Nauka", Leningrad, 146 p. (in Russian)
B.V. (1973).- Microfitofossilii Dokembriya Ukrainy [Microphytofossils from the Precambrian of the Ukraine].- Akademiya Nauk SSSR, Institut Geologii i Geokhronologii Dokembriya, Izdatel'stvo "Nauka", Leningrad, 58 p. + XL pl. (in Russian)
B.V., T.N. & N.S. (1976).- Mikrofitofossilii Dokembriya, Kembriya i Ordovika [Microphytofossils from the Precambrian, Cambrian and Ordovician].- Akademiya Nauk SSSR, Institut Geologii i Geokhronologii Dokembriya, Izdatel'stvo "Nauka", Leningrad, 106 p. (in Russian)
G.J.M. & P. (2004).- Resistant macromolecules of extant and fossil microalgae.- Phycological Research, Kochi, vol. 52, n° 4, p. 325-339.
G. (1976).- Late Precambrian microfossils from Visingsö Beds in southern Sweden.- Fossils Strata, Oslo, vol. 9, 57 p.
G. (1984).- The oldest eucaryotic cells.- Scientific American, New York, vol. 250, p. 48-57.
G. (1990).- Giant acanthomorph acritarchs from the upper Proterozoic in southern Norway.- Palaeontology, London, vol. 33, Part 2, p. 287-298.
D. (1962).- Evidence from recent plankton regarding the biological affinities of Tasmanites 1875 and Leiosphaeridia 1958.- Geological magazine, Cambridge, vol. 99, n° 4, p. 353-362.
S., A., L.A. G.L. & P.A. (2000).- Pseudoneochloris marina (Chlorophyta), a new coccoid ulvophycean alga, and its phylogenetic position inferred from morphological and molecular date.- Journal of Phycology, Oxford, vol. 36, n° 3, p. 596-604.
Y.Z. & Z.L. (1993).- The eukaryotic significance of microfossils from the Changcheng System.- Acta Micropaleontologica Sinica, Nanjing, vol. 10, p. 167-180.
T.V. (1982).- Microfossilii rifeya Yuzhnogo Urala [Microfossils of the Riphean of the South Urals].- In: B.M. (ed.), Stratotip Rifeya. Paleontologiya. Paleomagnetism [The Riphean stratotype, palaeontology, palaeomagnetism].- Akademiya Nauk SSSR, Ordena Trudovogo Krasnogo Znameni Geologicheskii Institut, Trudy, Moscow, n° 368, p. 84-120. (in Russian)
L., X., F. & J. (2005).- Protists of the Upper Mesoproterozoic Ruyang Group in Shanxi Province, China.- Precambrian Research, Amsterdam, vol. 141, n° 1-2, p. 49-66.
S. & H. (1995).- Megascopic multicellular organisms from the 1700-million-year-old Tuanshanzi Formation in the Jixian area, North China.- Science, Washington, vol. 270, n° 5236, p. 620-622.
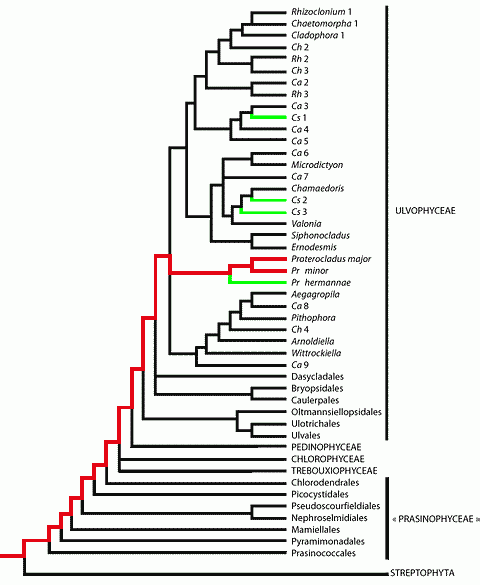
Click on thumbnail to enlarge the image.
Table I: Hypothesis concerning the location of a fossil Ulvophyceae from Svanbergfjellet, Proterocladus (ca. 750 Ma), in the phylogenetic tree of the Chlorophyta.
![]() The dendrogram of the
"Prasinophyceae" summarizes a more detailed one in et alii
(2004). The dendrogram of the "Neochlorophyta" (Ulvophyceae, Chlorophyceae, Trebouxiophyceae) is
derived mainly from
(1997), et alii
(1998a, 1998b), et alii
(2000), et alii
(2001), et alii
(2001) and et alii
(2001). As there is no agreement about the relative positions of these three classes, I present them as an unsolved trichotomy. The
placement of the Pedinophyceae near the Ulvophyceae is plausible but not demonstrated ( et alii,
2000; et alii,
2004). I assumed the traditional position of the Chlorodendrales
within the
"Prasinophyceae" because that location is in agreement with most dendrograms. However, on the phylogenetic tree of et alii
(2000) this clade is inserted between the Ulvophyceae and the cluster Chlorophyceae + Trebouxiophyceae, which
accords better with its ontogeny (development of a phycoplast).
The dendrogram of the
"Prasinophyceae" summarizes a more detailed one in et alii
(2004). The dendrogram of the "Neochlorophyta" (Ulvophyceae, Chlorophyceae, Trebouxiophyceae) is
derived mainly from
(1997), et alii
(1998a, 1998b), et alii
(2000), et alii
(2001), et alii
(2001) and et alii
(2001). As there is no agreement about the relative positions of these three classes, I present them as an unsolved trichotomy. The
placement of the Pedinophyceae near the Ulvophyceae is plausible but not demonstrated ( et alii,
2000; et alii,
2004). I assumed the traditional position of the Chlorodendrales
within the
"Prasinophyceae" because that location is in agreement with most dendrograms. However, on the phylogenetic tree of et alii
(2000) this clade is inserted between the Ulvophyceae and the cluster Chlorophyceae + Trebouxiophyceae, which
accords better with its ontogeny (development of a phycoplast).
![]() The red line shows the stages
that must be traversed in order to reach the position of Proterocladus, starting
from the last common ancestor of the Viridiplantae. The green line shows the paths from the morphotype of Cladophora (which is polyphyletic) to the morphotype of Cladophoropsis, which is repeatedly and
independently derived from ancestral morphs near Cladophora pellucidoidea, Valonia utricularis, Chamaedoris peniculum, Proterocladus major
or P. minor.
The red line shows the stages
that must be traversed in order to reach the position of Proterocladus, starting
from the last common ancestor of the Viridiplantae. The green line shows the paths from the morphotype of Cladophora (which is polyphyletic) to the morphotype of Cladophoropsis, which is repeatedly and
independently derived from ancestral morphs near Cladophora pellucidoidea, Valonia utricularis, Chamaedoris peniculum, Proterocladus major
or P. minor.
![]() As may be seen in this dendrogram,
Proterocladus (Ulvophyceae, Cladophorale) is separated by at least five nodal points from the point of origin of the Neochlorophyta.
Below this point seven more nodal points must be added to reach the level of the
common ancestor of the Viridiplantae.
As may be seen in this dendrogram,
Proterocladus (Ulvophyceae, Cladophorale) is separated by at least five nodal points from the point of origin of the Neochlorophyta.
Below this point seven more nodal points must be added to reach the level of the
common ancestor of the Viridiplantae.
![]() Abbreviations. Chaetomorpha 1: Ch. crassa, Ch. antennina –
Ch. 2: Ch. moniligera – Ch. 3:
Ch. linum – Ch. 4: Ch. okamurae
Abbreviations. Chaetomorpha 1: Ch. crassa, Ch. antennina –
Ch. 2: Ch. moniligera – Ch. 3:
Ch. linum – Ch. 4: Ch. okamurae
![]() Cladophora 1: Ca. rupestris - Ca. 2: Ca. albida, Ca. sericea, Ca. vagabunda, Ca. glomerata – Ca. 3: Ca. pellucida, Ca. sakaii, Ca. japonica – Ca. 4: Ca. pellucidoidea – Ca. 5: Ca. ohkuboana – Ca. 6: Ca. catenata, Ca. liebetruthii - Ca. 7: Ca. coelothrix, Ca. prolifera, Ca. socialis – Ca. 8: Ca. sp. – Ca. 9: Ca. conchopheria
Cladophora 1: Ca. rupestris - Ca. 2: Ca. albida, Ca. sericea, Ca. vagabunda, Ca. glomerata – Ca. 3: Ca. pellucida, Ca. sakaii, Ca. japonica – Ca. 4: Ca. pellucidoidea – Ca. 5: Ca. ohkuboana – Ca. 6: Ca. catenata, Ca. liebetruthii - Ca. 7: Ca. coelothrix, Ca. prolifera, Ca. socialis – Ca. 8: Ca. sp. – Ca. 9: Ca. conchopheria
![]() Cladophoropsis 1:
Cs. fasciculatus – Cs. 2: Cs. vaucheriaeformis – Cs. 3: Cs. membranacea
Cladophoropsis 1:
Cs. fasciculatus – Cs. 2: Cs. vaucheriaeformis – Cs. 3: Cs. membranacea
![]() Rhizoclonium 1:
Rh. grande – Rh. 2: Rh. sp. – Rh. 3: Rh. hieroglyphicum
Rhizoclonium 1:
Rh. grande – Rh. 2: Rh. sp. – Rh. 3: Rh. hieroglyphicum
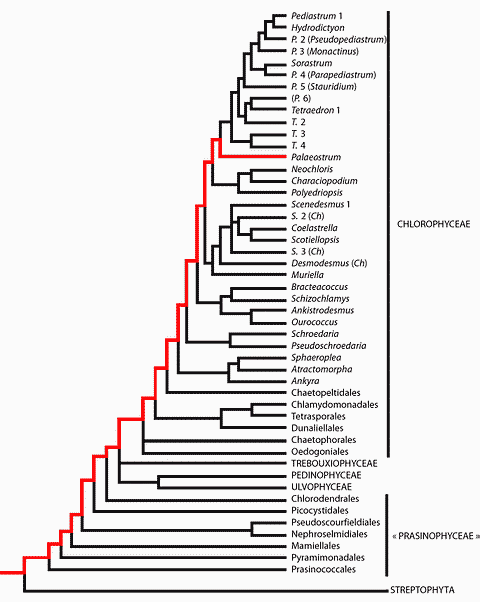
Click on thumbnail to enlarge the image.
Table II: Hypothesis concerning the location of a fossil Chlorophyceae from Svanbergfjellet, Palaeastrum (ca. 750 Ma), in the phylogenetic tree of the Chlorophyta.
![]() The red line shows the stages
that must be traversed in order to reach Palaeastrum, starting from the last common ancestor of the Viridiplantae.
The red line shows the stages
that must be traversed in order to reach Palaeastrum, starting from the last common ancestor of the Viridiplantae.
![]() As may be seen in this dendrogram, Palaeastrum (Chlorophyceae, Sphaeropleale akin to the Hydrodictyaceae) is separated by at least nine nodal points from the point of origin of the Neochlorophyta. Starting from this point in order to go back to the common ancestor of the Viridiplantae, seven more nodal
points must be added.
As may be seen in this dendrogram, Palaeastrum (Chlorophyceae, Sphaeropleale akin to the Hydrodictyaceae) is separated by at least nine nodal points from the point of origin of the Neochlorophyta. Starting from this point in order to go back to the common ancestor of the Viridiplantae, seven more nodal
points must be added.
![]() Abbreviations. Pediastrum 1:
P. angulosum, P. duplex – P. 2: P. boryanum, P. kawraiskyi –
P. 3: P. simplex – P. 4: P. biradiatum – P. 5: P. tetras, P. privum – (P. 6): "P. boryanum v. longicorne".
Abbreviations. Pediastrum 1:
P. angulosum, P. duplex – P. 2: P. boryanum, P. kawraiskyi –
P. 3: P. simplex – P. 4: P. biradiatum – P. 5: P. tetras, P. privum – (P. 6): "P. boryanum v. longicorne".
![]() Scenesdesmus 1: S. ovalternus, S. producto-capitatus – S. 2: S. ("Chlorella") vacuolatus - S. 3: S. obliquus, S. ("Chlorella") rubescens – S. 4: S. (" Chlorella ") abundans, S. costato-granulatus, S. communis, S. pupukensis.
Scenesdesmus 1: S. ovalternus, S. producto-capitatus – S. 2: S. ("Chlorella") vacuolatus - S. 3: S. obliquus, S. ("Chlorella") rubescens – S. 4: S. (" Chlorella ") abundans, S. costato-granulatus, S. communis, S. pupukensis.
![]() Tetraedron 1: T. minimum 1 – T. 2: T. minimum 2 - T. 3: T. caudatum, "Chlorotetraedron" bitridens, T. pentaedricum – T. 4: T. bitridens.
Tetraedron 1: T. minimum 1 – T. 2: T. minimum 2 - T. 3: T. caudatum, "Chlorotetraedron" bitridens, T. pentaedricum – T. 4: T. bitridens.
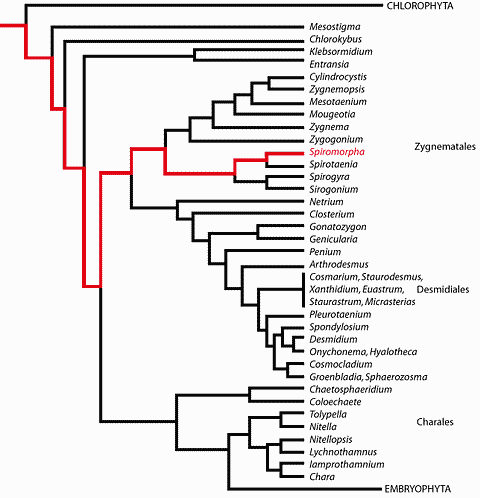
Click on thumbnail to enlarge the image.
Table III: Hypothesis concerning the location of a fossil Zygnematale from Ruyang, Spiromorpha (ca. 1200 Ma), in the phylogenetic tree of the Chlorophyta.
![]() The green line shows the stages
that must be traversed in order to reach Spiromorpha, starting from the last common ancestor of the Viridiplantae.
The green line shows the stages
that must be traversed in order to reach Spiromorpha, starting from the last common ancestor of the Viridiplantae.
![]() et alii
(2005) demonstrated that Spiromorpha (Ruyang, China, between 1200 and 1300 Ma) is closely akin to the recent Spirotaenia, itself akin
to Sirogonium and Spirogyra.
et alii
(2005) demonstrated that Spiromorpha (Ruyang, China, between 1200 and 1300 Ma) is closely akin to the recent Spirotaenia, itself akin
to Sirogonium and Spirogyra.
![]() The whole dendrogram of the Streptophyta was
constructed in accordance with that of et alii
(2001). The tree for the Zygnematophyceae is a combination of those of
&
(1999) and
et alii
(2001) regarding SSU rRNA and with that of et alii
(2000) regarding rbcL. &
(2005) have demonstrated that Cosmarium, Staurastrum
and Staurodesmus are polyphyletic and that the genera Euastrum
and Xanthidium are interspersed among several species of these three
"genera".
et alii
(2005) have clarified the phylogenetic tree of the
numerous species of
Spirogyra and Sirogonium. The alleged "Mesotaeniaceae" have no
true homogeneity. The Zygnematales show a clear-cut division into two groups: a
group with Zygnema and another group with Spirogyra. The Desmidiales are holophyletic. & classify
Spirogyra as a sister-group of a cluster Zygnematales + Desmidiales, whereas
and place
Spirogyra and its relatives near the stem of the Zygnematales.
The whole dendrogram of the Streptophyta was
constructed in accordance with that of et alii
(2001). The tree for the Zygnematophyceae is a combination of those of
&
(1999) and
et alii
(2001) regarding SSU rRNA and with that of et alii
(2000) regarding rbcL. &
(2005) have demonstrated that Cosmarium, Staurastrum
and Staurodesmus are polyphyletic and that the genera Euastrum
and Xanthidium are interspersed among several species of these three
"genera".
et alii
(2005) have clarified the phylogenetic tree of the
numerous species of
Spirogyra and Sirogonium. The alleged "Mesotaeniaceae" have no
true homogeneity. The Zygnematales show a clear-cut division into two groups: a
group with Zygnema and another group with Spirogyra. The Desmidiales are holophyletic. & classify
Spirogyra as a sister-group of a cluster Zygnematales + Desmidiales, whereas
and place
Spirogyra and its relatives near the stem of the Zygnematales.
![]() As we see from this table, Spiromorpha (ca. 1200 Ma) is very close to the base of the Zygnematophyceae: only three nodal points separate them. Starting
from the origin of the Zygnematophyceae, five nodal points must be added downward in order to reach the level of the last common ancestor of the
Viridiplantae.
As we see from this table, Spiromorpha (ca. 1200 Ma) is very close to the base of the Zygnematophyceae: only three nodal points separate them. Starting
from the origin of the Zygnematophyceae, five nodal points must be added downward in order to reach the level of the last common ancestor of the
Viridiplantae.
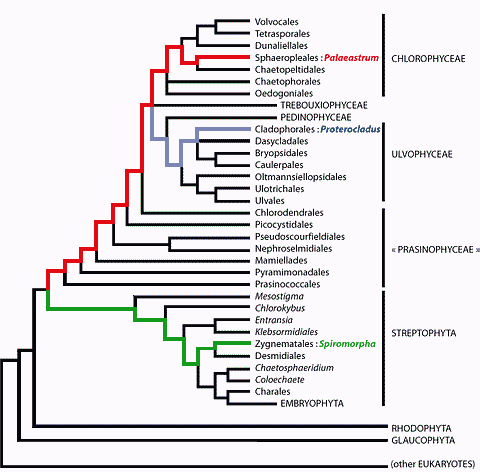
Click on thumbnail to enlarge the image.
Table IV: Hypothesis concerning the location of three fossil algae, Palaeastrum, Proterocladus and Spiromorpha, in the phylogenetic tree of the Plantae.
![]() This table is a
condensation of the three preceding dendrograms. The red line indicates the distance
traversed from the point of origin of the Neochlorophyta to Palaeastrum. The blue line: from the point of origin of the Neochlorophyta to Proterocladus. The purple line: from the point of origin of the Neochlorophyta to the last common ancestor of the Viridiplantae. The green line: from the point of origin of the Viridiplantae to Spiromorpha.
This table is a
condensation of the three preceding dendrograms. The red line indicates the distance
traversed from the point of origin of the Neochlorophyta to Palaeastrum. The blue line: from the point of origin of the Neochlorophyta to Proterocladus. The purple line: from the point of origin of the Neochlorophyta to the last common ancestor of the Viridiplantae. The green line: from the point of origin of the Viridiplantae to Spiromorpha.
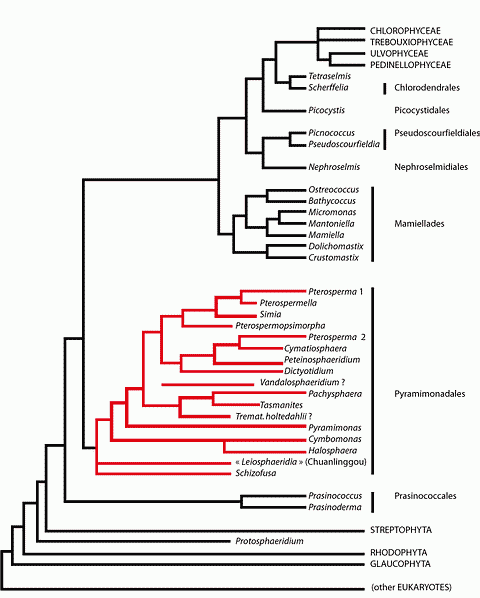
Click on thumbnail to enlarge the image.
Table V: Hypothesis concerning the location of some Precambrian Pyramimonadales in the phylogenetic tree of the Chlorophyta.
![]() This dendrogram
summarizes (with many simplifications) the phylogenetic tree that et alii
(2004) set up using the SSU rDNA of some planktonic
"Prasinophyceae" that are still living today. In a very hypothetical way I inserted some Precambrian fossils on the left of the table. The
location assigned them is only tentative. Concerning the Pedinophyceae and the Chlorodendrales, see the commentary on Table I
This dendrogram
summarizes (with many simplifications) the phylogenetic tree that et alii
(2004) set up using the SSU rDNA of some planktonic
"Prasinophyceae" that are still living today. In a very hypothetical way I inserted some Precambrian fossils on the left of the table. The
location assigned them is only tentative. Concerning the Pedinophyceae and the Chlorodendrales, see the commentary on Table I ![]() .
.
Click on thumbnail to enlarge the image.
Table VI: An example of the conflict between morphologic taxonomy and molecular phylogeny: the alleged "Chlorococcale" Neochloris.
![]() This dendrogram
is adapted from the phylogenetic tree of et alii
(2000) derived from the gene for SSU rRNA. The "genus" Neochloris
(1955) was originally assigned the highly polyphyletic group of the
"Chlorococcales". The molecular analysis shows that five species of the alleged
"genus" belong to four very distant clades and must be reassigned
among three classes of the Chlorophyta.
This dendrogram
is adapted from the phylogenetic tree of et alii
(2000) derived from the gene for SSU rRNA. The "genus" Neochloris
(1955) was originally assigned the highly polyphyletic group of the
"Chlorococcales". The molecular analysis shows that five species of the alleged
"genus" belong to four very distant clades and must be reassigned
among three classes of the Chlorophyta.
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Sphaeromorph acritarchs with a smooth envelope | |||||||
| Bothrioligotriletum exasperatum | O | ||||||
| Leioligotriletum crassum | La | ||||||
| Leioligotriletum nitidum | La | ||||||
| Mycteroligotriletum marmoratum | O | ||||||
| Nucellosphaeridium minutum | Be | ||||||
| Protosphaeridium acis | K | Be | |||||
| Protosphaeridium densum | La | Ik | K | ||||
| Protosphaeridium flexuosum | La | Su | Be | ||||
| Protosphaeridium laccatum | K | Be | |||||
| Protosphaeridium pallaceum | K | ||||||
| Protosphaeridium parvulum | La | ||||||
| Protosphaeridium patelliforme | Be | ||||||
| Protosphaeridium rigidulum | La | K | |||||
| Protosphaeridium scabridum | Be | ||||||
| Protosphaeridium tuberculiferum | Be | ||||||
| Protosphaeridium sp. | La | Car | Su | K | Be | ||
| Stenozonoligotriletum sokovii | La | ||||||
| Stenozonoligotriletum validum | La | ||||||
| Stictosphaeridium pectinale | Ik | ||||||
| Stictosphaeridium sinapticuliferum | A | K | |||||
| Trachyoligotriletum asperatum | O | ||||||
| Trachyoligotriletum laminaritum | Sa | ||||||
| Trematosphaeridium holtedahlii | Bu | ||||||
| Colonial coccoid Cyanobacteria (cf. Chroococcaceae) | |||||||
| Favososphaeridium sp. | A | ||||||
| Gloeocapsomorpha sp. | Be | ||||||
| Polyedrosphaeridium bullatum | Ik | ||||||
| Protoleiosphaeridium conglutinatum | O | ||||||
| *Incertae sedis | |||||||
| Synsphaeridium conglutinatum | Car | ||||||
Table VII: Microfossils from Russian sites dated between 1800 and 2000 Ma.
![]() These fossils (see
& ,
1992) were extracted by and his followers after maceration in hydrofluoric acid.
Today, with few exceptions the biopolymers of unicellular Eukaryotes that resist acetolysis are
synthesized only by the Viridiplantae and the Dinoflagellates. With regard to the
Prokaryotes, the part fossilized most commonly is the envelope of colonial
Cyanobacteria. With the exception of the Acritarchs, the paleontological sites of Russia dated from 1800 to 2000 Ma have yielded
nothing but fossils of colonial coccoid Cyanobacteria (or of Synsphaeridium, inc. sed.) protected by their envelope. On the contrary, at sites of the same
age-range outside Russia, Eukaryotes are exceedingly rare and bacteria predominate because the paleontologists of the
"American school" have studied sedimentary rocks by serial section instead of treating them
with hydrofluoric acid as the
"Russian school" did (, 1984).
These fossils (see
& ,
1992) were extracted by and his followers after maceration in hydrofluoric acid.
Today, with few exceptions the biopolymers of unicellular Eukaryotes that resist acetolysis are
synthesized only by the Viridiplantae and the Dinoflagellates. With regard to the
Prokaryotes, the part fossilized most commonly is the envelope of colonial
Cyanobacteria. With the exception of the Acritarchs, the paleontological sites of Russia dated from 1800 to 2000 Ma have yielded
nothing but fossils of colonial coccoid Cyanobacteria (or of Synsphaeridium, inc. sed.) protected by their envelope. On the contrary, at sites of the same
age-range outside Russia, Eukaryotes are exceedingly rare and bacteria predominate because the paleontologists of the
"American school" have studied sedimentary rocks by serial section instead of treating them
with hydrofluoric acid as the
"Russian school" did (, 1984).
1. La = Ladoga Formation, ca 2000 Ma.
2. lk = Ikabijk ,"ca 2200 Ma"; Car = Carelian Complex, "ca 2100 Ma" (the dating of these two sites seems too high,
so they have been referred to group 1).
3. A = Ayan, ca 2000 Ma; Bu = Butun ca 1950 Ma.
4. O = Onega Fm., ca 1900 Ma; Sa = Sakuhan Fm., ca 1900 Ma; Su = Sujsari Complex, ca 1900 Ma.
5. K = Krivoj-Rog, ca 1870 Ma.
6. Be = Besovets, ca 1800 Ma.
All of these datings are from (1966,
1969, 1973) or from
et alii (1976).