Contents
[Introduction] [Materials and methods]
[Results] [Discussion] and ...
[Bibliographic references]
Department of Microbiology, University of Massachusetts, N.
Pleasant St., Morrill IV North, room 203, Amherst, MA 01003 (U.S.A.)
Center for Integrative Geosciences, University of
Connecticut, 354 Mansfield Road, U-2045, Storrs, CT 06269 (U.S.A.); Laboratory
for Orthopaedic Biomechanics (LOB), Biozentrum/Pharmazentrum, University of
Basel, Klingelbergstrasse 50-70, CH-4056 Basel (Switzerland)
Center for Integrative Geosciences, University of
Connecticut, 354 Mansfield Road, U-2045, Storrs, CT 06269 (U.S.A.)
Manuscript online since December 10, 2008
Microbial mats are synergistic microbial consortia through which major elements, including sulfur, are cycled due to microbial and geological processes. Depth profiles of pH, O2, sulfide, exopolymeric substances (EPS), and the rate of sulfate reduction were determined in an Oscillatoria sp. and Microcoleus-dominated marine microbial mat at the Great Sippewissett salt marsh, Massachusetts. In addition, measurements in spirochete enrichments and Spirochaetae litoralis cultures showed sulfide consumption during which polysulfides, thiosulfate, and presumably sulfate formed. These data suggest that spirochetes can play a role in the cycling of sulfur in these mats. The obligate to facultative anaerobic spirochetes may consume sulfide to remove oxygen. Furthermore, spirochetes may enhance preservation of microbial mats within the rock record by degrading EPS and producing low molecular weight organic compounds (LMWOC). Both sulfide oxidation (i.e., oxygen removal) and EPS degradation (i.e., production of LMW organic compounds) stimulate the activity of sulfate-reducing bacteria (SRB), which are responsible for the precipitation of calcium carbonate in most lithifying mats.
Sulfur cycle; spirochetes; microbial mat; EPS; oxygen detoxification; rock record; calcification.
E.A., O. & P.T. (2008).- Spirochetes and salt marsh microbial mat geochemistry: Implications for the fossil record.- Carnets de Géologie / Notebooks on Geology, Brest, Article 2008/09 (CG2008_A09)
Spirochetes et géochimie des tapis microbiens de marais salants : Implications pour l'enregistrement fossile.- Les tapis microbiens sont des associations microbiennes agissant en synergie par le biais desquelles les éléments majeurs tels que le soufre sont recyclés au travers de processus microbiens et géologiques. Les variations de pH, de l'O2, des sulfures, des substances exopolymériques (EPS) et du taux de réduction des sulfates ont été mesurées en fonction de la profondeur dans un tapis microbien marin dominé par une Oscillatoria sp. et des Microcoleus, tapis localisé dans les marais salants de Great Sippewissett, Massachusetts. De plus, des mesures de l'enrichissement de spirochètes ainsi que des cultures de Spirochaetae litoralis ont mis en évidence la consommation des sulfures et la production concomitante de polysulfures, de thiosulfate et probablement aussi de sulfates. Ces données suggèrent que les spirochètes peuvent jouer un rôle dans le cycle du soufre dans ces tapis microbiens. Les spirochètes, qui sont des organismes obligatoirement ou facultativement anaérobies, pourraient utiliser les sulfures pour éliminer l'oxygène de leur environnement. Ainsi les spirochètes pourraient aussi favoriser la préservation des tapis microbiens dans l'enregistrement sédimentaire en dégradant les EPS et en libérant des composés organiques de faible poids moléculaire (LMWOC). L'oxydation des sulfures (c'est-à-dire suppression de l'oxygène) et la dégradation des EPS (c'est-à-dire la production de matériaux de faible poids moléculaire) stimulent tous deux l'activité des bactéries sulfato-réductrices (SRB) qui sont responsables de la précipitation du carbonate de calcium dans la plupart des tapis microbiens en cours de lithification.
Cycle du soufre ; spirochetes ; tapis microbien ; substances exopolymériques ; détoxination de l'oxygène ; enregistrement sédimentaire ; calcification.
Microbial mats are sedimentary biofilms, which are cohesive structures consisting of microorganisms and the organic-inorganic matrix in which they live. The biotic component of the mat is highly productive, dynamic and interactive. Within only a few millimeters, microbes participate in diagenetic reactions involving metals and cycle major elements ( & , 1993; & van , 1993; et alii, 1994; & , 1994; , 1995). Lithified microbial mats or microbialites (including stromatolites) may persist in the rock record and have a laminated structure similar to that of modern (non-lithified) mats. Some of these ancient microbial mats have been dated to 3.8 Ga (, 1988) and represent the Earth's earliest known life (, 1967; et alii, 1993; , 2000; , 2008). The Earth's first terrestrial life is believed to have arisen from microbial mats that have been dated to be approximately 2.6 billion years ago ( et alii, 2000). Fossilized microbial mats are useful in determining the ancient Earth's environment, stratigraphy and paleocurrent direction (, 1967; et alii, 1980).
Microbial metabolism alters the saturation index (SI) of calcium carbonate ( & , 2005) thereby facilitating precipitation or dissolution ( & , 1992; & , 2005). Geochemical processes (such as degassing) may also accomplish SI changes, but are believed to play a less important role ( & , 2005). The precipitation of calcium carbonate, which takes place within the EPS matrix ( & , 2005) may provide the opportunity for microbial mat fossilization (, 1979; , 1996; et alii, 2005; , 2007). In order to understand the mechanism of preservation of the mats, it is necessary to study these underlying key microbial processes. This allows inferences to be made about ancient microbial mats and their environments based on modern mat structure, microbial communities and their metabolic processes (, 1993; et alii, 1996; et alii, 2001).
Cyanobacteria, sulfur-reducing bacteria (SRB), sulfide-oxidizing bacteria (SOB) and anoxyphototrophs are well studied (van , 1993; , 1995), but aerobic and anaerobic heterotrophs have received much less attention. This group of organisms plays, however, a critical role in the degradation of organic carbon, particularly that of large molecules. Spirochetes are a group of anaerobic heterotrophic bacteria with a distinct morphology: A long, thin, wavy, thread-like appearance with flagella inside the periplasmic space (, 1978; et alii, 2000; , 2000). They are abundant in most natural ecosystems, including the human mouth, animal digestive tract, fresh water and anoxic sediments ( & , 1984). Numerous spirochete morphotypes have been observed within mats using microscopy and their presence has been confirmed using molecular identification methods, since spirochetes have unique ribosomal RNA ( et alii, 1990; et alii, 2006; et alii, 2006). Spirochetes may swim through relatively viscous media (up to 500 centipoises) as compared with other flagellated bacteria ( & , 1977; et alii, 2000). In general, spirochetes are resistant to the bacterial RNA polymerase inhibitor, rifampin, which has been employed as a tool for spirochete selection ( & , 1980; & , 1981) and allows for selective enrichment techniques (see below).
Spirochetes residing in mats have to be tolerant of the extremely high sulfide levels and have been proposed to oxidize sulfide for metabolic processes and/or oxygen detoxification within the mat ( et alii, 2004). Spirochetes, as a group, have a wide range of tolerance to oxygen. The group includes aerobes, facultative aerobes, microaerobic and obligate anaerobic members ( et alii, 2007). Furthermore, they are able to produce a variety of metabolic end-products, such as ethanol, lactate, succinate, carbon dioxide or acetate from larger molecules ( et alii, 1999). This research focused on two goals: The first goal was to elucidate the role of microbial mat spirochetes, in relation to sulfide, within the synergistic microbial consortia of the marine microbial mat. The second goal was to develop a model that incorporated the spirochetes' activity within the mat, especially with respect to EPS degradation, co-metabolism with other mat organisms and the formation of calcium carbonate for mat lithification. The current study investigates the contribution of spirochetes in the Great Sippewissett Salt Marsh (GSSM) in sulfur cycling and implies a role of these heterotrophs in providing SRB with LMWOC during their fermentative metabolism, thereby playing a critical role in the lithification of mats ( et alii, 2007).
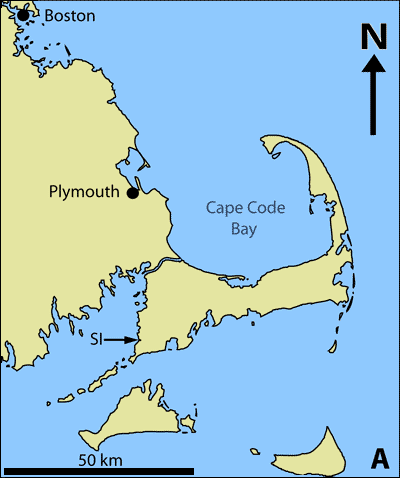
The Great Sippewissett Salt Marsh is located on Cape Cod, Massachusetts (41°35'30"N, 70°38'50"W) (Fig.
1.A-B ![]() ) ( et alii,
1987; et alii,
1998; et alii,
2008). The marsh is protected by sand dunes from wave activity and is flooded with an influx of marine water with the tide. The intertidal mats have been the focus of numerous biogeochemical investigations ( et alii,
1987; et alii,
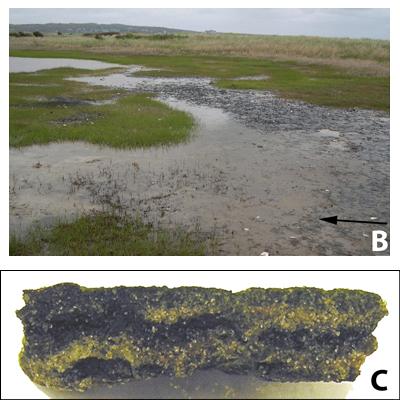
2008). The mats are laminated, primarily due to the decreasing light quality and quantity with depth, resulting in spatial separation of microorganisms engaging in different metabolic processes. The resulting laminae appear as characteristically green, pink and black layers, which reflect the inherent microbial and chemical differences (Fig.
1.C
) ( et alii,
1987; et alii,
1998; et alii,
2008). The marsh is protected by sand dunes from wave activity and is flooded with an influx of marine water with the tide. The intertidal mats have been the focus of numerous biogeochemical investigations ( et alii,
1987; et alii,
2008). The mats are laminated, primarily due to the decreasing light quality and quantity with depth, resulting in spatial separation of microorganisms engaging in different metabolic processes. The resulting laminae appear as characteristically green, pink and black layers, which reflect the inherent microbial and chemical differences (Fig.
1.C ![]() )
( et alii, 1987).
These sandy microbial mats of the Sippewissett salt marsh are similar to the
mats previously described, and the chemical stratification in the mat is due to
microbial metabolism that creates steep pH, oxygen and sulfide gradients that
fluctuate diurnally ( et alii, 1987). Most GSSM spirochetes have not yet been cultivated and their structural, physiological and metabolic features remain elusive, particularly with respect to their role in microbial mat geochemistry.
)
( et alii, 1987).
These sandy microbial mats of the Sippewissett salt marsh are similar to the
mats previously described, and the chemical stratification in the mat is due to
microbial metabolism that creates steep pH, oxygen and sulfide gradients that
fluctuate diurnally ( et alii, 1987). Most GSSM spirochetes have not yet been cultivated and their structural, physiological and metabolic features remain elusive, particularly with respect to their role in microbial mat geochemistry.
 |
 |
Click on thumbnail to enlarge the image.
Figure 1: The Great Sippewissett Salt Marsh is labeled SI on the map, which shows the salt marsh's location on Cape Cod, Massachusetts (A). Sandy microbial mats, such as these describe in this article are found throughout the salt marsh. The arrow points to the surface of the cyanobacteria-rich, uppermost mat layer (B). The microbial mat in cross-section shows the laminae, which reflect a changing chemistry and microbial community with depth (C). The mat sample is approximately 1 cm thick.
Mat samples (stored on ice) were obtained during the spring and summer months of 2004-2006 using sterile techniques. Mat samples for microelectrode analyses, microscopy and inoculation of enrichment cultures were maintained in microcosms (15 x 15 cm plastic trays), moistened regularly with sterile water and exposed to normal diel cycles. Phase-contrast microscopy was performed using an Olympus (BX50) microscope with an Olympus camera (U-CMAD-2, Olympus America, Japan). Images were captured using the program MagnaFire 2.1.
The mat samples were dissected into three layers, distinguished by color: A green top layer, a middle pink layer and a bottom black layer. The sulfide concentration was measured in each layer using a sulfidostat ( and , 2004), which allows for the simultaneous measurement of sulfide and oxygen in a sealed chamber at a specific temperature. Steady state sulfide concentration was measured in triplicate for each 0.25 gram mat sample at 25° C and 0% oxygen under a flow of nitrogen gas and the results were averaged. Sterile mat sand served as a negative control.
Sulfide, oxygen and pH microelectrode profiles and sulfate reduction rates were measured in situ in August 2004 and in the laboratory in July 2006 to verify that the geochemical measurements of laboratory samples were consistent with field measurements. The vertical depth profiles were measured in triplicate every 200-250 μm increment using a combination needle oxygen/pH electrode (Diamond General, Ann Arbor, MI) and ion-selective sulfide needle electrode (Microscale Measurements, The Netherlands). The oxygen concentration was recorded with a picoammeter (Unisense PA2000, Denmark), and the pH and sulfide concentration with a high-impedance millivolt meter (Microscale Measurements, The Netherlands) ( et alii, 1991). Sulfide readings were combined with the pH measurements to calculate the exact sulfide concentration.
Sulfate reduction rates were measured over eight millimeters horizontally for approximately the top ten millimeters of mat with a 35SO42--sulfate labeled silver foil method ( et alii, 2000). A pixel pattern, formed when 35SO42- is reduced to 35S2- and then precipitates to Ag35S on the foil surface, is visualized by radiography ( et alii, 2006). Mat sections were incubated in the field for four hours.
Polysulfides, polythionates and thiosulfate were measured spectrophotometrically following cyanolysis at pH 8.7 and 4.8, respectively ( et alii, 1990; & van , 1993). Briefly, samples were incubated in a 0.2 M KCN solution, during which sulfane-sulfur forms thiocyante (SCN-), which reacts with Fe(III) nitrate and is measured colorimetrically.
Pigments were analyzed by spectrophotometry (λ = 360-900 nm). Each layer was weighed and freeze-dried overnight in the dark. Pigments were extracted in the dark for one hour with methanol. The presence of chlorophyll a (Chla), bacteriochlorophyll a (BChla) and elemental sulfur were determined spectrophotometrically (van et alii, 1985).
The EPS concentrations were determined for each mat layer after extraction in 100 mM EDTA using agitation and sonication. After extraction, EPS was precipitated by adding two volumes cold ethanol, recovered by centrifugation and air-dried. Alcian blue was added to bind to the EPS resulting in an inverse relationship between absorption and the EPS concentration ( & , 1995; et alii, 2005). A standard curve was prepared with xanthane, and EPS concentration was determined spectrophotometrically at λ = 614.5 nm ( & , 1995; et alii, 2005).
Spirochete enrichments were prepared in a modified Marine Broth medium (Difco 2216, pH 7.3 supplemented with glucose [0.028 M] and Na2S [8.3 mM]). The glucose and the 0.22 μm filter-sterilized sodium sulfide solution were added separately using sterile, anaerobic techniques. The medium was made anoxic by gassing with a N2/CO2 (20:80) mixture for one hour at room temperature. Ten milliliters of the modified Marine Broth medium were anaerobically transferred to Hungate culture tubes and augmented with rifampin (10 μg/ml final concentration). The cultures were maintained at this anoxic state and strict anaerobic technique was carefully followed when sampling the cultures.
A microbial mat slurry was prepared for inoculation of spirochete enrichments by mixing approximately three grams of mat material and 20 milliliters of the medium. The slurry was filtered using Whatman (no. 40) paper, one milliliter of the filtrate was transferred to each tube, then the tubes were incubated in the dark at 30° C. The approximate percentage of spirochetes in the enrichment cultures was estimated by counting cells during microscopic observations.
S. litoralis (strain 2029), obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ), was grown on modified Marine Broth medium, amended with NaS2 [2.08 mM] and glucose. S. litoralis was also grown on S. litoralis medium containing cysteine [2.85 mM]. S. litoralis is a strictly anaerobic spirochete isolated from marine sediment and is used in this research as a positive control for spirochete growth ( & , 1970).
The GSSM mats are distributed in a patchy pattern, exposed to air and inundated with channels supplying marine water. Although the mat typically consists of three distinct layers: Green, pink and black; in the field either the green or pink layer may be absent, perhaps due to the impact of shade, organic debris or overlaying water.
Using microscopy, a plethora of eukaryotic and prokaryotic microorganisms were observed, including numerous diatoms, radiolaria, cyanobacteria, spirilla, the phototrophic purple-sulfur bacterium Thiocapsa sp., spirochetes and numerous other bacteria of various morphologies. The top layer is dominated by the filamentous cyanobacteria Oscillatoria sp. and Microcoleus chthonoplastes. Motile spirochete morphologies were identified using Nomarsky microscopy and found to be present throughout the mat, especially in the micro-oxic (pink) and anoxic (black) layers. Various spirochete morphotypes were observed, ranging from those with larger diameters (~0.4 μm) and wider wavelengths to very tightly coiled smaller diameter (~0.1 μm) spirochetes.
Geochemical analyses of the mat are consistent with similar analyses for microbial mats sampled elsewhere ( et alii, 1991; & van , 1993; van , 1993; et alii, 1999; et alii, 2002; et alii, 2004; et alii, 2005).
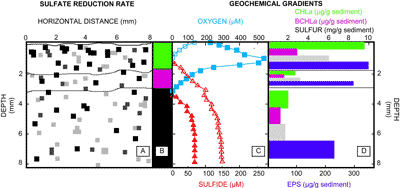
Mapping of sulfate reduction rates using silver foil (Fig.
2.A ![]() ) showed that the highest rates occur in the uppermost portion of the upper mat layer (ca. 2 mm) (the approximate depth of which is indicated in
Fig. 2.B
) showed that the highest rates occur in the uppermost portion of the upper mat layer (ca. 2 mm) (the approximate depth of which is indicated in
Fig. 2.B ![]() ). Oxygen profiles for both light and dark conditions
(Fig. 2.C
). Oxygen profiles for both light and dark conditions
(Fig. 2.C ![]() )
are consistent with diel cyanobacterial activity resulting in oxygen penetration to a greater depth (i.e., 3.5 mm) during daylight hours, peaking at ca. 230 μM O2 saturation at 1.5 mm depth. During night, oxygen is present only in the upper millimeter. The sulfide profile shows that the concentration increases with depth to ca. 150 μm and that the nighttime interface between oxygen and sulfide (ca. 0.8 mm) is close to the surface. Microcosm depth profiles displayed identical distribution patterns and similar concentrations as measured in situ. The concentrations of Chla, BChla and elemental sulfur all decreased with depth
(Fig. 2.D
)
are consistent with diel cyanobacterial activity resulting in oxygen penetration to a greater depth (i.e., 3.5 mm) during daylight hours, peaking at ca. 230 μM O2 saturation at 1.5 mm depth. During night, oxygen is present only in the upper millimeter. The sulfide profile shows that the concentration increases with depth to ca. 150 μm and that the nighttime interface between oxygen and sulfide (ca. 0.8 mm) is close to the surface. Microcosm depth profiles displayed identical distribution patterns and similar concentrations as measured in situ. The concentrations of Chla, BChla and elemental sulfur all decreased with depth
(Fig. 2.D ![]() ). Similarly, the amount of EPS decreased with depth, indicating consumption of these polymers below the green cyanobacterial layer (Fig.
2.D
). Similarly, the amount of EPS decreased with depth, indicating consumption of these polymers below the green cyanobacterial layer (Fig.
2.D ![]() ).
).
Click on thumbnail to enlarge the image.
Figure 2: Sulfate reduction rate approximations (panel A) are reported with depth for each of the green, pink and black layers (top to bottom) presented in panel B. Squares in panel A indicate the presence of Ag235S; the higher sulfate reduction rates are depicted by darker pixels. The panel represents a two-dimensional map of sulfate-reduction. Horizontal distance measured is indicated on the x-axis of panel A, and depth in millimeters is reported on the y-axes for all panels. Microelecrode depth profiles of oxygen and sulfide are shown in panel C: Oxygen (blue squares) and sulfide (red triangles) for both light and dark conditions are represented by closed and open symbols, respectively. Geochemical depth profiles are shown in panel D per layer as determined by panel B for (top to bottom): Chlorophyll a (green), bacteriochlorophyll a (pink), elemental sulfur (gray), and exopolymeric substances (EPS) (blue). The varying thickness of the bars in panel D is reflective of the difference in thickness between the mat layers.
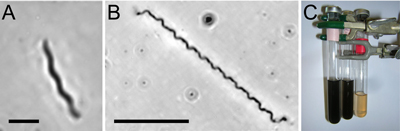
Spirochete enrichments contained at least two spirochete morphotypes: A relatively shorter spirochete, approximately 0.4 μm in diameter (Fig.
3.A ![]() )
and a thinner, more tightly coiled spirochete (Fig. 3.B
)
and a thinner, more tightly coiled spirochete (Fig. 3.B ![]() ). Both of these spirochete morphotypes displayed expected spirocheatal swimming motion as they translated through the liquid medium. The FeS that was visible in the uninoculated medium disappeared after two weeks in the spirochete enrichment cultures, at which point the medium was yellowish in color (Fig.
3.C
). Both of these spirochete morphotypes displayed expected spirocheatal swimming motion as they translated through the liquid medium. The FeS that was visible in the uninoculated medium disappeared after two weeks in the spirochete enrichment cultures, at which point the medium was yellowish in color (Fig.
3.C ![]() ), indicative of polysulfide formation. S. litoralis cultures displayed a similar loss of FeS (data not shown).
), indicative of polysulfide formation. S. litoralis cultures displayed a similar loss of FeS (data not shown).
Click on thumbnail to enlarge the image.
Figure 3: (A & B) Two representatives of spirochete morphotypes observed in spirochete enrichment cultures (bar = 10 μm). (C) Uninoculated modified marine broth medium (far left tube) and spirochete enrichment culture at five days (middle tube) and fifteen days (far right tube). The formation of polysulfides is indicated by the yellowish hue (far right tube). The volume in the far right tube is less than the others because this tube was photographed immediately following sampling. The head space in all cultures was maintained anoxic.
After a brief lag phase, the cultures decreased in sulfide concentration by 0.23 mM and 0.15 mM, in ca. 5 days when growing on modified marine broth medium and S. litoralis medium, respectively
(Table 1 ![]() ). Similarly, sulfide consumption started in enrichments after a 3-5 day lag phase, after which approximately 80-90% of the enrichment consisted of spirochete morphotypes and the other 10-20% of the culture consisted of rod-shaped bacteria
(Table 1
). Similarly, sulfide consumption started in enrichments after a 3-5 day lag phase, after which approximately 80-90% of the enrichment consisted of spirochete morphotypes and the other 10-20% of the culture consisted of rod-shaped bacteria
(Table 1 ![]() ). These spirochete enrichments consumed ca. 0.64 mM sulfide over the following ten days
(Table 1
). These spirochete enrichments consumed ca. 0.64 mM sulfide over the following ten days
(Table 1 ![]() ). Polysulfides and thiosulfates, but not polythionates, were produced in enrichments and cultures, coinciding with sulfide oxidation
(Table 1
). Polysulfides and thiosulfates, but not polythionates, were produced in enrichments and cultures, coinciding with sulfide oxidation
(Table 1 ![]() ).
).
Table 1: Sulfide consumption and product formation in spirochete enrichments and S. litoralis cultures grown on either modified marine broth or S. litoralis medium.
Lag phase before sulfide consumption started, average sulfide consumption and average final concentrations of
polysulfide, thiosulfate and polythionates are reported.
*when sulfide consumption commenced, MMB = modified marine broth, SLM
= S. litoralis medium, NM = not measured, ND = not detected (1 μM).
The microbial mat is a dynamic system in which microorganisms survive and thrive under extreme diel redox fluctuations (de et alii,
1989). The close collaboration of microbes in consortia enables successful adaptation to these unusual conditions (van ,
1993).
Within the mat, chemical stratification and the resulting microbial community
stratification (Fig. 2 ![]() )
occur due to the oxic / anoxic gradient. This is different than, but contributes
to, the lamination that is observed between the mat layers. Mat layer lamination
is a function of the variation between the microbial community of each layer, as
compared to another layer, and the changing upward displacement of these
stratified communities (Fig.
1.C
)
occur due to the oxic / anoxic gradient. This is different than, but contributes
to, the lamination that is observed between the mat layers. Mat layer lamination
is a function of the variation between the microbial community of each layer, as
compared to another layer, and the changing upward displacement of these
stratified communities (Fig.
1.C ![]() ). Furthermore, through microbial metabolic activity, the geochemical environment is continuously altered, potentially creating the conditions for calcium carbonate production ( & ,
2005) and ultimately mat preservation. Consequently, modern microbial mats may serve as an analogue of ancient mats and provide information on the antiquity of life and the environment of ancient Earth.
). Furthermore, through microbial metabolic activity, the geochemical environment is continuously altered, potentially creating the conditions for calcium carbonate production ( & ,
2005) and ultimately mat preservation. Consequently, modern microbial mats may serve as an analogue of ancient mats and provide information on the antiquity of life and the environment of ancient Earth.
Precipitation of calcium carbonate in mats is common in tropical environments with carbonate sediments ( et alii, 2000; et alii, 2004; et alii, 2006), but recently carbonate precipitation was also documented in a siliclastic mat in a temperate climate ( et alii, 2008) similar to the GSSM. Two key microbial processes are involved in CaCO3 production ( & , 2005): (1) alteration of saturation and (2) degradation of EPS, liberating bound calcium and creating nucleation sites. Our observations imply that spirochetes may play an indirect but important role in both processes.
An alternative mechanism for the preservation of microbial mats, microbial-induced sedimentary structures (MISS), must be discussed since the degradation of EPS also reduces the likelihood of microfossil preservation through the preservation of cyanobacterial EPS ( et alii, 2001). MISS preservation occurs because the relationship between sedimentation and microbial growth and activity allows for the "biostabalization" of mat sediment and the opportunity for subsequent preservation of the microbial mat ( et alii, 1995). The role of EPS and the continued preservation of microbial mats even when EPS levels are relatively low has been observed when fossilized microbial mats, including stromatolites, originating from the Proterozoic and the Phanerozoic eons were compared ( et alii, 2006).
Early Proterozoic mats contained cyanobacterial EPS at relatively high concentrations, due to the lower number of grazing and burrowing organisms that, if present in abundant numbers, would have consumed the EPS ( et alii, 2006). Mats like the 1.6 billion year old Chorhat Sandstone, Vindhyan Supergroup, M.P, India were preserved, in part, due to the abundant EPS ( et alii, 2006). In contrast, their Phanerozoic counterparts are relatively EPS-poor, due to increased predation on mat organisms and consumption of EPS, yet mat preservation occurs due to "biostabilization" of the mat sediment, the evolution and subsequent occurrence of metazoans and perhaps calcium carbonate lithification via the above-mentioned processes (, 1970).
Spirochetes are found in all layers of the mat, instead of being confined to one particular region, and have been observed using microscopy (this study; et alii, 2005). Furthermore, they are motile through the viscous EPS matrix of the mat ( & , 1977; et alii, 2000) and have the metabolic capacity to degrade complex polysaccharides ( & , 1982) that form the bulk of EPS (, 2000; et alii, 2007). Despite their motility, spirochetes prefer a microaerophilic or anoxic environment.
The decrease in EPS with depth (Fig.
2.D ![]() )
perhaps resulting, in part, from spirochete degradation of EPS is one property that allows us to speculate about the role of spirochetes in mats. Particularly, spirochetes may stimulate the activity of SRB, which are significant in calcium carbonate precipitation ( et alii,
1984; et alii,
1998, 2000; et alii,
2006). SRB have been shown to be most abundant in the uppermost layers of other salt marsh mats ( et alii,
1992, 1999). Metabolism of SRB produces HCO3- and increases the alkalinity, thereby changing the saturation index to favor precipitation of carbonate minerals. High levels of sulfides are toxic to SRB ( et alii,
2006) and
the removal of sulfide not only benefits spirochetes, but also SRB. In contrast to most SOB, the motility of spirochetes enables these organisms to migrate to the depth horizon where both sulfide and an electron acceptor (i.e., oxygen or nitrate) ( et alii,
1998; et alii,
2006) exist. The persistence of sulfide consumption in spirochete enrichment cultures that were treated with
rifampin, to limit the contribution of other prokaryotic organisms that may otherwise consume sulfide, suggests that spirochetes are at least partially responsible for the oxidation of sulfide. It should be mentioned, however, that rifampin is a prokaryotic polymerase inhibitor, and there are eukaryotic heterotrophs and perhaps even some prokaryotic organisms that were not inhibited by the addition of rifampin to the mat samples or cultures.
)
perhaps resulting, in part, from spirochete degradation of EPS is one property that allows us to speculate about the role of spirochetes in mats. Particularly, spirochetes may stimulate the activity of SRB, which are significant in calcium carbonate precipitation ( et alii,
1984; et alii,
1998, 2000; et alii,
2006). SRB have been shown to be most abundant in the uppermost layers of other salt marsh mats ( et alii,
1992, 1999). Metabolism of SRB produces HCO3- and increases the alkalinity, thereby changing the saturation index to favor precipitation of carbonate minerals. High levels of sulfides are toxic to SRB ( et alii,
2006) and
the removal of sulfide not only benefits spirochetes, but also SRB. In contrast to most SOB, the motility of spirochetes enables these organisms to migrate to the depth horizon where both sulfide and an electron acceptor (i.e., oxygen or nitrate) ( et alii,
1998; et alii,
2006) exist. The persistence of sulfide consumption in spirochete enrichment cultures that were treated with
rifampin, to limit the contribution of other prokaryotic organisms that may otherwise consume sulfide, suggests that spirochetes are at least partially responsible for the oxidation of sulfide. It should be mentioned, however, that rifampin is a prokaryotic polymerase inhibitor, and there are eukaryotic heterotrophs and perhaps even some prokaryotic organisms that were not inhibited by the addition of rifampin to the mat samples or cultures.
Sulfide oxidation occurs in the spirochaeta component of the "Thiodendron" consortium presumably during H2O2 detoxification ( et alii, 2004). A similar process, but for the purpose of detoxifying oxygen from the spirochetes' microenvironment, is suggested here. Sulfide consumption was measured in spirochete enrichment cultures under anoxic conditions, where possible electron acceptors included nitrate and sulfate. However, within the microbial mat oxygen and sulfide concentrations are in a state of flux and sulfide and oxygen may overlap within a given area. It is this dynamic chemistry that makes it necessary for spirochetes to possess the means of tolerating and altering their environment to survive. If the consumption of sulfide were coupled to oxygen, this would further stimulate SRB metabolism. Finally, SRB rely on small organic compounds, of low molecular weight, for their energy metabolism. These organic compounds, such as acetic acid, can be produced by spirochetes during fermentation ( & , 1981). Of particular importance is the above-mentioned capacity of spirochetes to degrade complex polysaccharides ( & , 1982), and by extension, possibly EPS.
The presence of abundant, highly-motile mat spirochetes, where EPS comprises a major carbon reservoir ( et alii, 2005), suggests that EPS may indeed be an important carbon source for these microorganisms. If this assumption is correct, the role of spirochetes in CaCO3 precipitation can be extended. As indicated previously (et alii, 2001; & , 2005), EPS degradation is required in order for CaCO3 to precipitate, and this is a role that spirochetes may play in mat lithification. Fresh EPS effectively binds cations, including Ca2+, and inhibits precipitation. Ca2+ is released and nucleation sites are created within the biofilm matrix, processes which allow calcium carbonate to be precipitated, only after EPS is degraded. Overall, EPS is degraded, releasing calcium to precipitate out as calcium carbonate. Our model of a spirochete-SRB synergy is not novel. A symbiotic spirochete-SRB consortium, in which the former organism was thought to provide fermentation products to furnish a carbon source for the latter, was described in a deep-sea oligochaete ( et alii, 2005).
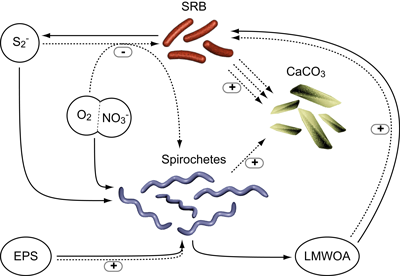
Click on thumbnail to enlarge the image.
Figure 4: Conceptual model of the role of spirochetes in microbial mat biogeochemistry. Through sulfide removal (using oxygen or nitrate) and degradation of exopolymeric substances (EPS), spirochetes stimulate sulfate-reducing bacteria (SRB), thereby enhancing long-term preservation of microbial mats through calcium carbonate precipitation. EPS degradation by spirochetes also results in calcium liberation and formation of nucleation sites for CaCO3 precipitation. Solid lines indicate flow of materials, dotted lines the interactions between reservoirs (sulfide, EPS, oxygen, nitrate, low-molecular weight organics (LMWOA) and calcium carbonate crystals) and microorganisms (spirochetes and SRB, respectively). "+" and "-" represent stimulatory and inhibitory interactions, respectively.
Our model (Fig. 4 ![]() ) identifies spirochetes as important heterotrophs linking cyanobacteria and "terminal" anaerobic heterotrophs, such as
SRB. Although there are most certainly other heterotrophic organisms that contribute to this process, it is feasible that spirochetes contribute to the nutritional requirements of SRB and
thus indirectly to the formation of calcium carbonate. Through this microbial interaction and directly by degrading EPS, spirochetes may play a role in the preservation of microbial mats in the rock record. Although this research suggests that spirochetes
are at least somewhat significant in the fossilization of microbial mats, this preliminary
study has raised numerous questions that will be answered only by continued research. Thus, future work must be completed to determine if, indeed, spirochetes have a significant role in this process, determine
just how significant their role is and specifically what it is that spirochetes do within the mat and in relation to other microorganisms. One aspect of the future work will focus on estimating spirochete abundance
in these mats. Spirochete abundance may be statistically estimated using Chao1 once 16S rRNA sequences have been derived from this mat. Nitrate, sulfate, fumarate, and Fe (III) possibly serve as electron acceptors when spirochetes oxidize sulfide. Additionally, future research will focus on determining which electron acceptors are used
to perform this process.
) identifies spirochetes as important heterotrophs linking cyanobacteria and "terminal" anaerobic heterotrophs, such as
SRB. Although there are most certainly other heterotrophic organisms that contribute to this process, it is feasible that spirochetes contribute to the nutritional requirements of SRB and
thus indirectly to the formation of calcium carbonate. Through this microbial interaction and directly by degrading EPS, spirochetes may play a role in the preservation of microbial mats in the rock record. Although this research suggests that spirochetes
are at least somewhat significant in the fossilization of microbial mats, this preliminary
study has raised numerous questions that will be answered only by continued research. Thus, future work must be completed to determine if, indeed, spirochetes have a significant role in this process, determine
just how significant their role is and specifically what it is that spirochetes do within the mat and in relation to other microorganisms. One aspect of the future work will focus on estimating spirochete abundance
in these mats. Spirochete abundance may be statistically estimated using Chao1 once 16S rRNA sequences have been derived from this mat. Nitrate, sulfate, fumarate, and Fe (III) possibly serve as electron acceptors when spirochetes oxidize sulfide. Additionally, future research will focus on determining which electron acceptors are used
to perform this process.
We would like to thank the following people, departments and agencies for their support of this research: Celeste , Suphan , Michael , Susan , Michelle , James , Lynn , Kristin , Margaret , Dennis , Chris , The University of Massachusetts Graduate School and the Departments of Microbiology and Geosciences, the University of Connecticut Department of Marine Science at Avery Point, the Marine Biological Laboratory, Woods Hole, MA and the NASA Planetary Biology Internship Program. We also express our appreciation to all of the reviewers for suggestions that improved our article.
G., A. & J. (2001).- Photosynthesis-induced biofilm calcification and calcium concentrations in Phanerozoic oceans.- Science, Washington, vol. 292, n° 5522, p. 1701-1704.
L.K., R.P., C., A.W., D.H., J.R., K.M. & P.T. (2006).- Sulfate reducing bacteria in microbial mats: Changing paradigms, new discoveries.- Sedimentary Geology, Amsterdam, vol. 185, n° 3-4, p. 131-145.
B.M., S.P., D.J., M., T., F., T.M., M., L.L., R.M., S.R., L.E., C., M. & K. (2002).- Long-term manipulations of intact microbial mat communities in a greenhouse collaboratory: Simulating Earth's present and past field environments.- Astrobiology, New Rochelle, vol. 2, n° 4, p. 383-402.
B.M., H.W., J.E., D.E. & D.J. (1994).- Nitrogen cycling in microbial mat communities: The quantitative importance of N-fixation and other sources of N for primary productivity. In: L.J. & P. (eds.), Microbial mats: Structure, development and environmental significance.- Springer Verlag, Berlin, p. 265-272.
A., C., R. & N. (2005).- Coexistence of bacterial sulfide oxidizers, sulfate reducers, and spirochetes in a gutless worm (Oligochaeta) from the Peru margin.- Applied and Environmental Microbiology, Washington, vol. 71, n° 3, p. 1553–1561.
C., K. & M. (2005).- Quantification of single-species marine biofilm with alcian blue.- The Journal of Young Investigators, Washington, vol. 12.
O., A.W., C., C., K. & P.T. (2007).- Exopolymeric substances of sulfate-reducing bacteria: Interactions with calcium at alkaline pH and implication for formation of carbonate minerals.- Geobiology, Oxford, vol. 5, n° 4, p. 401-411.
D.H., L.K. & P.T. (2008).- Vertical distribution of methane metabolism in microbial mats of the Great Sippewissett Salt Marsh.- Environmental Microbiology, Oxford, vol. 10, n° 4, p. 967-977.
D.E. & D.J. (1993).- Biogeochemical cycles of carbon, sulfur, and free oxygen in a microbial mat.- Geochimica et Cosmochimica acta, Amsterdam, vol. 57, n° 16, p. 3971-3984.
L., M.L., Z., C.M., D., C., W., R.E., J. & P.T. (2005).- Community structure, geochemical characteristics and mineralogy of a hypersaline microbial mat, Cabo Rojo, PR.- Geomicrobiology Journal, Philadelphia, vol. 22, n° 6, p. 269-281.
H.S. & C. (1992).- Bacterially induced lithification of microbial mats.- PALAIOS, Lawrence, vol. 7, n° 3, p. 277-293.
J.D., T., D.J. & D.R. (1998).- Carbohydrate oxidation coupled to Fe(III) reduction, a novel form of anaerobic metabolism.- Anaerobe, Amsterdam, vol. 4, n° 6, p. 277-282.
A.W. (2000).- Exopolymer microdomains as a structuring agent or heterogeneity within microbial biofilms. In: R.E. & S.M. (eds.), Microbial sediments.- Springer, Amsterdam, p. 9-15.
A.W., P.T. & R.P. (2005).- Production and cycling of natural microbial exopolymers (EPS) within a marine stromatolite.- Paleogeography, Palaeogeography, Palaeoclimatology, Amsterdam, vol. 219, n° 1-2, p. 71-86.
D.J. (1995).- The biogeochemistry of hypersaline microbial mats. In: J.G. (ed.), Advances in microbial ecology.- Plenum Press, New York, vol. 14, p. 251-74.
G.A., M.Y. & Y.Y. (2004).- The role of oxygen in the regulation of the metabolism of aerotolerant spirochetes, a major component of "Thiodendron" bacterial sulfur mats.- Mikrobiologiia, Moskva, t. 73, n° 6, p. 725-733; Microbiology, New York, vol. 73, n° 6, p. 621-628.
C. & P.T. (2005).- Microbial lithification in marine stromatolites and hypersaline mats.- Trends in Microbiology, London, vol. 13, n° 9, p. 429-438.
C., P.T., L.K. & R.P. (2004).- Microbe-mineral interactions: Early carbonate precipitation in a hypersaline lake (Eleuthera Island, Bahamas).- Sedimentology, Oxford, vol. 51, p. 745-765.
M., S., E., K.-H. & E. (eds.) (2007).- The Prokaryotes.- Springer, Berlin, 3rd ed., Vols. 1-7 (Set), 7000 p.
H.L. (1996).- How microbes influence mineral growth and dissolution.- Chemical Geology, Amsterdam, vol. 132, n° 1-4, p. 5-9.
P. (1970).- Phanerozoic stromatolites: Noncompetitive ecologic restriction by grazing and burrowing animals.- Science, Washington, vol. 169, n° 3941, p.171-173.
H. van (1993).- Microbial mats: A joint venture.- Marine Geology, Amsterdam, vol. 113, n° 1-2, p. 3-25.
H. van, E., J. & R. (1985).- Diel cycle of metabolism of phototrophic purple sulfur bacteria in Lake Cisó (Spain).- Limnology and Oceanography, Waco, vol. 30, n° 5, p. 932-943.
E.P. & E. (1977).- Motility of flagellated bacteria in viscous environments.- Journal of Bacteriology, Washington, vol. 132, n° 1, p. 356-358.
C.S. & E. (1981).- Branched-chain amino acid fermentation by a marine spirochete: Strategy for starvation survival.- Journal of Bacteriology, Washington, vol. 148, n° 1, p. 109-116.
C.S. & E. (1984).- Ecology of spirochetes.- Annual Review of Microbiology, Palo Alto, vol. 38, p. 161-192.
R.B. & E. (1970).- Spirochaeta litoralis sp. n., a strictly anaerobic marine spirochete.- Archives of Microbiology, Berlin, vol. 74, n° 1, p. 1-18.
P. (1967).- Stromatolites: Use in stratigraphic correlation and paleocurrent determination.- Science, Washington, vol. 157, n° 3792, p. 1043-1045.
S.C. (1978).- Anatomy and chemistry of spirochetes.- Microbiology Review, Washington, vol. 42, n° 1, p. 114-160.
A., P. & H.N. (2006).- Anaerobic sulfide oxidation with nitrate by a freshwater Beggiatoa enrichment culture.- Applied and Environmental Microbiology, Washington, vol. 72, n° 7, p. 4755–4760.
B., J. & L.J. (2008).- Calcium carbonate precipitation in cyanobacterial mats from sandy tidal flats of the North Sea.- Geobiology, Oxford, vol. 6, n° 1, p. 46-56.
W.E. (1979).- Calcification by bacteria and algae. In: P.A. & D.J. (eds.), Calcification by bacteria and algae.- Scientific Publishing Company, Amsterdam, p. 612.
J.R., T.M., J.R. & J.A. (1999).- Acetogenesis from H2 plus CO2 by spirochetes from termite guts.- Science, Washington, vol. 283, n° 5402, p. 686-689.
S.B. & E. (1980).- Rifampin as a selective agent for isolation of oral spirochetes.- Journal of Clinical Microbiology, vol. 12, n° 6, p. 792-795.
S.B., B.J. & E. (2000).- Free-living saccharolytic spirochetes: The genus Spirochaeta. In: M., S., E., K.-H. & E. (eds.), The Prokaryotes.- Springer, Berlin, vol. 7, p. 195-210.
R., J., J., J.R., S.R., B., J.A., D.A., M.L. & N.R. (2006).- Unexpected diversity and complexity of the Guerrero Negro hypersaline microbial mat.- Applied and Environmental Microbiology, Washington, vol. 72, n° 5, p. 3685-3695.
W.B., D.T., M.E., H.E. & P.B. (1984).- Calcification of cyanobacterial mats in Solar Lake, Sinai.- Geology, Boulder, vol. 12, n° 10, p. 623-626.
L. (2000).- Spirochetes. In: Encyclopedia of microbiology.- Academic Press, London, p. 353-363.
L., E.S., D., S., D., S., S. & J. (1980).- The microbial community in the layered sediments at Laguna Figueroa, Baja California, Mexico – Does it have Precambrian analogs?- Precambrian Research, Amsterdam, vol. 11, n° 2, p. 93-123.
L., J.O., M. & D.J (1990).- In: L., J.O., M. & D.J (eds.) Handbook of Protoctistia: The structure, cultivation, habitats and life histories of the eukaryotic microorganisms and their descendants exclusive of animals, plants and fungi: A guide to the algae, ciliates, foraminifera, sporozoa, water molds, slime molds and other protoctists.- Jones and Bartlett Publishers, Boston.
L., A. & M. (1998).- Cosmopolitan distribution of the large composite microbial mat spirochete, Spirosymplokos deltaeiberi.- International Microbiology, Barcelona, vol. 1, n° 1, p. 27-34.
S.J., G., K.D., T.M., A.P. & C.R.L. (1996).- Evidence for life on Earth by 3800 million years ago.- Nature, London, vol. 384, n° 6604, p. 55-59.
K.H. & D. (1994).- Iron and manganese in anaerobic respiration: Environmental significance, physiology, and regulation.- Annual Review of Microbiology, Palo Alto, vol. 48, p. 311-343.
J., J.F. & B.K. (1987).- Structure of a microbial mat at Great Sippewissett Salt Marsh, Massachusetts.- FEMS Microbiology Letters, Oxford, vol. 45, n° 6, p. 343-364.
N., G., T. & W.E. (1995).- M.I.S.S. Microbially induced sedimentary structures.- In: "Sediment 95", Freiberg (24-28 Mai 1995).
N., G., T. & W.E. (2001).- Microbially induced sedimentary structures: A new category within the classification of primary sedimentary structures.- Journal of Sedimentary Research, Tulsa, vol. 71, n° 5, p. 649-656.
U. & A.L. (1995).- A dye-binding assay for the spectrophotometric measurement of transparent exopolymer particles (TEP).- Limnology and Oceanography, Waco, vol. 40, n° 7, p. 1326-1335.
B.J. & E. (1982).- Physiological diversity of rumen spirochetes.- Applied and Environmental Microbiology, Washington, vol. 43, n° 3, p. 686-693.
B.J., F.E., W.G., L.A., G.J., R.B., T.B., L., L. & C.R. (1991).- Phylogenetic analysis of the spirochetes.- Journal of Bacteriology, Washington, vol. 173, n° 19, p. 6101–6109.
B.K., A. & G.L. (1987).- Pigments, light penetration, and photosynthetic activity in the multi-layered microbial mats of Great Sippewissett Salt Marsh, Massachusetts.- FEMS Microbiology Letters, Oxford, vol. 45, n° 6, p. 365-376.
R.P., P.T., A.W., J.F., B.M., C., I.G., H.W., J.L., L., T.F., & D.J. (2000).- The role of microbes in accretion, lamination and early lithification of modern marine stromatolites.- Nature, London, vol. 406, n° 6799, p. 989-992.
R.E. (2000).- Microbial carbonates: The geological record of calcified bacterial-algal mats and biofilms.- Sedimentology, Oxford, vol. 47, n° 1, p. 179–214.
S., P.K., & P.G. (2006).- An example of microbial mat influences on clastic sedimentation: The Neoproterozoic Sonia Sandson, Rajasthan, India.- Journal of Earth Systems Science, Bangalore, vol. 115, n° 1, p. 49–60.
M. (1988).- A 3,800 million-year isotopic record of life of life from carbon in sedimentary rocks.- Nature, London, vol. 333, n° 6171, p. 313-318.
J.W. (1993).- Microfossils of the early Archean Apex chert: New evidence of the antiquity of life.- Science, Washington, vol. 260, n° 5108, p. 640-646.
D.G. & M.A. (2004).- Hydrogen sulfide consumption measured at low steady state concentrations using a sulfidostat.- Analytical Biochemistry, San Diego, vol. 324, n° 2, p. 269-275.
M. (2008).- Palaeontology: Modern life in ancient mats.- Nature, London, vol. 452, n° 7183, p. 40-41.
C., R., J.A., P.T., A.G. & Y. (2006).- Lithifying microbial mats in Lagoa Vermelha, Brazil: Modern Precambrian relics?- Sedimentary Geology, Amsterdam, vol. 185, n° 3-4, p. 175-183.
P.T. (2007).- Microbial carbonates: Bacterial metabolism, exopolymeric secretions and communication?- In: 4th EGU General Assembly.- Geophysical Research Abstracts, Katlenburg-Lindau, vol. 9, 02538, 2 p.
P.T., B.M. & R.P. (2000).- Microscale observations of sulfate reduction: Correlation of microbial activity with lithified micritic laminae in modern marine stromatolites.- Geology, Boulder, vol. 28, n° 10, p. 919-922.
P.T., J. & H. van (1991).- In situ characterization of sediments: Measurements of oxygen and sulfide profiles with a novel combined needle electrode.- Limnology and Oceanography, Waco, vol. 36, n° 7, p. 1476-1480.
P.T., M.R. & B.F. (1992).- Enumeration of bacteria which cleave or demethylate dimethylsulfoniopropionate in the Caribbean Sea.- Marine Ecology Progress Series, Oldendorf/Luhe, vol. 89, p. 293-296.
P.T. & H. van (1993).- Sulfur cycling in laminated marine ecosystems. In: R.S. (ed.), Biogeochemistry of global change: Radiatively active trace gasses.- Chapman Hall, New York p. 672-693.
P.T., R.F. & E.R. (1999).- Low-molecular-weight sulfonates, a major substrate for sulfate reducers in marine microbial mats.- Applied and Environmental Microbiology, Washington, vol. 65, n° 8, p. 3272–3278.
P.T., J.W. & H. van (1990).- Polysulfide utilization by Thiocapsa roseopersicinal.- Archives of Microbiology, Berlin, vol. 155, n° 1, p. 75-81.
P.T., R.P., , B.M., , S.E., I.G. & J.A. (1998).- Formation of lithified micritic laminae in modern marine stromatolites.- The American Mineralogist, Washington, vol. 83, n° 11-12, p. 1482-1493.
P.T. & J. (2005).- Microbial mats as bioreactors: Populations, processes, and product.- Paleogeography, Palaeogeography, Palaeoclimatology, Amsterdam, vol. 219, n° 1-2, p. 87-100.
Y., J.E. & H. (2000).- Geochemical evidence for terrestrial ecosystems 2.6 billion years ago.- Nature, London, vol. 408, n° 6812, p. 574-578.
F.H. & E.P. (1981).- Rifampin as a selective agent for the enumeration and isolation of spirochetes from salt marsh habitats.- Current Microbiology, New York, vol. 5, n° 5, p. 303-306.
R. de, H.M. & H. van (1989).- In situ fluctuations of oxygen and sulfide in marine microbial sediment ecosystems.- Netherlands Journal of Sea Research, vol. 23, n° 3, p. 271-281.