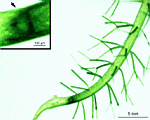
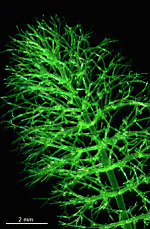
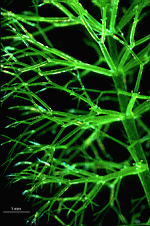
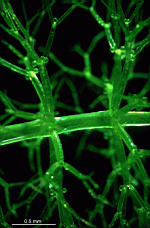
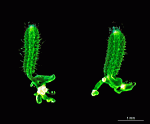Photo-Atlas of living Dasycladales
_
Sigrid Berger
Systematics and age of the Dasycladales
The order Dasycladales of the green algae includes macroalgae which grow in the shallow waters of tropical and subtropical shores as far north as the Mediterranean Sea. They are rarely found in deep water but have been observed to a depth of 20 m.
Dasycladales are now classified as members of the Class Ulvophyceae (Graham et Wilcox, 2000), although van den Hoek et alii (1993), due to their unique combination of features, placed them in a discrete class, the Dasycladophyceae.
Dasycladales are unicellular algae which can grow to a length of 200 mm in the major dimension. They may have been even larger in past geological times. This is an enormous size for a unicell. Multicellular organisms gain stability by constructing walls of differentiated cells to demarcate their several functional elements, while some of the huge unicellular organisms gain stability by surrounding themselves with a calcareous coating. This is how the long unicellular Dasycladales gain
enough stability to grow upright. When a calcified alga dies the coating may be preserved in the sediment as an impression of a once-living structure. Consequently, fossils of these coated algae exist, sometimes in great numbers, over relatively long periods of time. One of these long-enduring groups is the algal order called Dasycladales. It had already been in existence a long time at the dawn of the Cambrian period, about 570 million years ago. The fact that the Dasycladales have survived for so long a time with but few changes in distinctive features has led us to call them "living fossils".
Five families with nearly 200 genera, some with a large number of species, have evolved but most have become extinct. They flourished in the Middle Permian, the Early Jurassic, at the Jurassic/Cretaceous boundary and during the Palaeocene. In the Holocene new genera developed which, together with the genus Acetabularia that originated in earliest Oligocene times and some persistent Cretaceous genera, constitute the present-day representatives of the Order Dasycladales. It comprises two families, the Dasycladaceae and Polyphysaceae, with 10 genera and 38 species. Some genera are represented by only one or two species, and some species are rarely observed today.
Systematic classification
Section: Chlorophyta
 Class: Ulvophyceae (Dasycladophyceae)
Class: Ulvophyceae (Dasycladophyceae)

 Order: Dasycladales Pascher 1931
Order: Dasycladales Pascher 1931


 Family: Dasycladaceae Kützing 1843
Family: Dasycladaceae Kützing 1843
Genera: Batophora J. Agardh 1854, Bornetella Munier-Chalmas 1877, Cymopolia Lamouroux 1816, Chlorocladus Sonder 1871, Dasycladus C. Agardh 1828, Neomeris Lamouroux 1816
Species: Batophora occidentalis (Harvey 1858) S. Berger et Kaever ex M.J. Wynne 1998
 B. oerstedi J. Agardh 1854
B. oerstedi J. Agardh 1854
 Bornetella nitida (Harvey 1857) Munier-Chalmas 1877
Bornetella nitida (Harvey 1857) Munier-Chalmas 1877
 B. oligospora Solms-Laubach 1893
B. oligospora Solms-Laubach 1893
 B. sphaerica (Zanardini 1878) Solms-Laubach 1893
B. sphaerica (Zanardini 1878) Solms-Laubach 1893
 B. capitata (Harvey 1857) J. Agardh 1886
B. capitata (Harvey 1857) J. Agardh 1886
 Cymopolia barbata Lamouroux 1816
Cymopolia barbata Lamouroux 1816
 C. vanbosseae Solms-Laubach 1893
C. vanbosseae Solms-Laubach 1893
 Chlorocladus australasicus Sonder 1871
Chlorocladus australasicus Sonder 1871
 Dasycladus densus Womersley 1955
Dasycladus densus Womersley 1955
 D. ramosus Chamberlain 1958
D. ramosus Chamberlain 1958
 D. vermicularis (Scopoli) Krasser 1898
D. vermicularis (Scopoli) Krasser 1898
 Neomeris annulata Dickie 1875
Neomeris annulata Dickie 1875
 N. bilimbata Koster 1937
N. bilimbata Koster 1937
 N. cokeri Howe 1904
N. cokeri Howe 1904
 N. dumetosa Lamouroux 1816
N. dumetosa Lamouroux 1816
 N. mucosa Howe 1909
N. mucosa Howe 1909
 N. stipitata (Church 1895) Howe 1909
N. stipitata (Church 1895) Howe 1909
 N. vanbosseae (Sonder 1871) Howe 1909
N. vanbosseae (Sonder 1871) Howe 1909


 Family: Polyphysaceae Kützing (1843: 302, 311)
Family: Polyphysaceae Kützing (1843: 302, 311)


 [formerly Acetabulariaceae Nägeli (1847: 158, 252)]
[formerly Acetabulariaceae Nägeli (1847: 158, 252)]
Genera: Acetabularia J.V. Lamouroux (1812: 185), Chalmasia Solms-Laubach (1895: 32), Halicoryne Harvey (1859: 333), Parvocaulis S. Berger et alii (2003: 559)
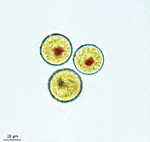
Species: Acetabularia acetabulum (Linnaeus) P.C. Silva (1952: 255)
 A. caliculus J.V. Lamouroux in Quoy et Gaimard (1824: 621, pl. 90, figs. 6, 7)
A. caliculus J.V. Lamouroux in Quoy et Gaimard (1824: 621, pl. 90, figs. 6, 7)
 A. crenulata J.V. Lamouroux (1816: 249, pl. 8, fig. 1)
A. crenulata J.V. Lamouroux (1816: 249, pl. 8, fig. 1)
 A. dentata Solms-Laubach (1895: 23, pl. 1, fig. 11)
A. dentata Solms-Laubach (1895: 23, pl. 1, fig. 11)
 A. farlowii Solms-Laubach (1895: 27, pl. 3, fig. 1)
A. farlowii Solms-Laubach (1895: 27, pl. 3, fig. 1)
 A. kilneri J. Agardh (1886: 171-172)
A. kilneri J. Agardh (1886: 171-172)
 A. major Martens (1866: 25, pl. 4, fig. 3)
A. major Martens (1866: 25, pl. 4, fig. 3)
 A. peniculus (R. Brown ex Turner) Solms-Laubach (1895: 27-28, pl. 2, figs. 2 & 6-7)
A. peniculus (R. Brown ex Turner) Solms-Laubach (1895: 27-28, pl. 2, figs. 2 & 6-7)
 A. ryukyuensis Okamura et Yamada in Okamura [1932:71(68), pl.285, figs. 5-12]
A. ryukyuensis Okamura et Yamada in Okamura [1932:71(68), pl.285, figs. 5-12]
 A. schenckii Möbius (1899: 318-320, pl. 10, figs. 8-12)
A. schenckii Möbius (1899: 318-320, pl. 10, figs. 8-12)
 Chalmasia antillana Solms-Laubach ( 1895: 32, pl. 3, figs. 2-3 & 5)
Chalmasia antillana Solms-Laubach ( 1895: 32, pl. 3, figs. 2-3 & 5)
 Halicoryne spicata (Kützing) Solms-Laubach (1895: 31-32, pl. 4, figs. 3, 7 ,9 & 11)
Halicoryne spicata (Kützing) Solms-Laubach (1895: 31-32, pl. 4, figs. 3, 7 ,9 & 11)
 H. wrightii Harvey (1859: 333)
H. wrightii Harvey (1859: 333)
 Parvocaulis clavata (Yamada) S. Berger et alii, comb. nov. (2003: 559)
Parvocaulis clavata (Yamada) S. Berger et alii, comb. nov. (2003: 559)
 P. exigua (Solms-Laubach) S. Berger et alii, comb. nov. (2003: 559)
P. exigua (Solms-Laubach) S. Berger et alii, comb. nov. (2003: 559)
 P. parvula (Solms-Laubach) S. Berger et alii, comb. nov. (2003: 559)
P. parvula (Solms-Laubach) S. Berger et alii, comb. nov. (2003: 559)
 P. polyphysoides (P. Crouan et H. Crouan in Schramm et Mazé) S. Berger et alii, comb. nov. (2003: 559)
P. polyphysoides (P. Crouan et H. Crouan in Schramm et Mazé) S. Berger et alii, comb. nov. (2003: 559)
 P. pusilla (M. Howe) S. Berger et alii, comb. nov. (2003: 560)
P. pusilla (M. Howe) S. Berger et alii, comb. nov. (2003: 560)
Morphology
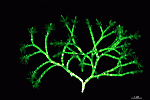
All Dasycladales have a similar and distinctive plan of construction. They are unicellular and radially symmetrical; although branching may occur and branched laterals are formed, no cell walls interrupt the continuity of the cytoplasm throughout the whole alga.
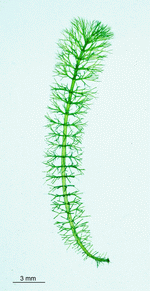
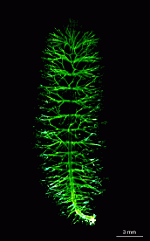
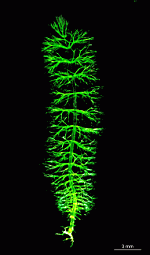
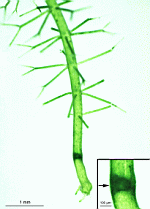
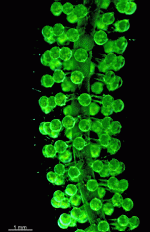
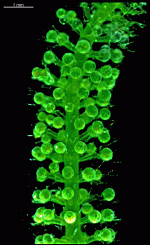
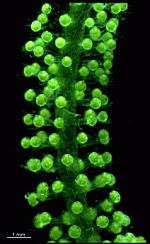
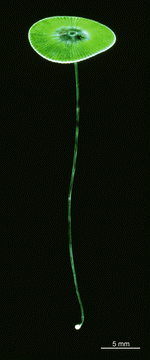
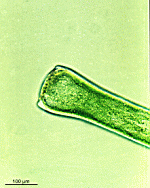
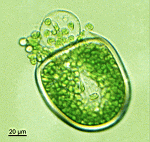
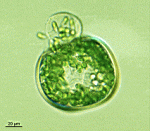
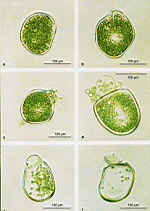
The main axis has a branched rhizoid at the base. This serves as a holdfast to a solid substratum. The main axis grows from its apical tip. The tip flattens periodically forming circularly arranged, branched laterals called whorls. The older whorls fall off leaving scars on the main axis. The length of time that the laterals persist varies according to species. Also the whorls may be modified in different ways according to species. In the genera Bornetella, Cymopolia and Neomeris of the family Dasycladaceae the laterals expand and fuse along their widened sides to form a cortex. Within the laterals, or in structures which develop from the laterals, gametangia may be formed. These structures are called gametophores and have a strict species-specific morphology.
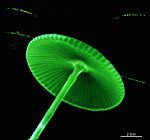
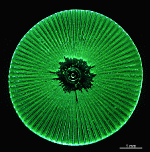
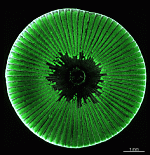
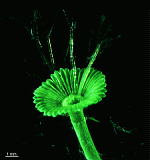
The two living families of the Dasycladales are easy to distinguish by their gametophores or fertile whorls. In the Dasycladaceae the fertile whorls are branched laterals. Ovoid or spherical gametophores are attached to the branches by means of a short stalk. In the family Polyphysaceae the fertile whorls are composed of unbranched broad laterals with special basal parts, called corona superior and corona inferior, the latter being found only in the genus Acetabularia. In most species of the Polyphysaceae the fertile laterals are close together giving the impression of a cap composed of rays sited on the apex of the main axis.
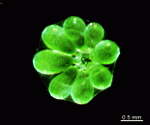
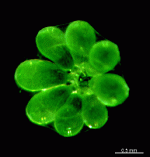
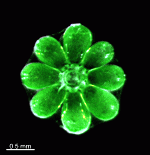
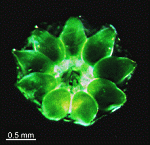
Species may exhibit a certain amount of variability, primarily in size but also in color. These variations may be due in part to the amount of light and the availability of nutrients. Also in the family Polyphysaceae the number of cap rays may vary to a certain degree. In particular, Acetabularia crenulata is highly variable in the shape of the cap rays. This led to confusion when the species was described in the literature. Each of the cap rays of A. crenulata always has a spine at its distal end. Cap rays of one cap may have spines that extend far out (one extreme) while the cap rays of another cap may have their spines embedded in the indented cap ray (the other extreme). However, there are many transitions. The variations are demonstrated in the several photographs of the cap rays of A. crenulata.
Life cycle
The life cycle starts with the growth of a diploid zygote. The zygote forms a branched rhizoid at the pole where the nucleus is located. Because the rhizoid fastens to a substratum this location of the nucleus ensures the possibility of regeneration when the rest of the cell is torn or grazed upon. At the opposite pole the zygote elongates and growth of the tip starts. After several days of growth, the tip flattens, and circularly arranged whorls of branched laterals are formed. Whorl formation is then followed by renewed tip growth. These events alternate periodically.
While calcium carbonate is deposited extracellularly around the main axis, the thin-walled tip and young laterals are free of calcification and thus exposed allow absorption of light and ingestion of nutrients.
These first stages of development are identical for all Dasycladales. Differences occur during the morphogenesis,
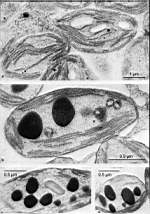
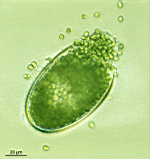
particularly during reproduction. Ovoid or spherical, short-stalked gametophores are formed on the laterals of the species belonging to the family Dasycladaceae. In the family Polyphysaceae the gametophores have the shape of cap-like structures formed by broad laterals. As soon as the gametophores are fully grown, the diploid (primary) nucleus in the rhizoid undergoes meiosis followed by many mitoses which result in a large number of haploid secondary nuclei. These secondary nuclei together with the cytoplasm containing plastids migrate up the main axis into the gametophores where each nucleus along with some cytoplasm is enclosed by a cell membrane and a cell wall to form the gametangium. An exception exists in the genera Cymopolia and Dasycladus where no gametangia form in the gametophores, for the gametophores themselves serve as gametangia.
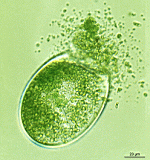
The secondary nucleus in the gametangium undergoes many mitoses. At this point the bleached mother cell is nearly devoid of cytoplasm and disintegrates, so the gametangia tumble out and sink to the substrate.
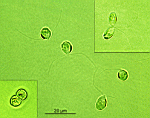
The gametangia are very thick-walled, operculate cysts that can survive a long time. When environmental conditions are favourable, a final division of the secondary nuclei occurs and gametes are formed with the nucleus located beneath the flagellar pole. The thick-walled cysts open by mean of a preformed lid, and the gametes swim out. Each gamete has two flagella of identical length and an eyespot which enables it to swim toward light (positively phototactic). The gametes look identical but are of different mating types. One gametangium releases gametes of only one type (+ or -), but one cell releases both types of gametes. Attracted by light, one + and one – gamete copulate at their flagellar poles and then fuse along their sides. The phototactically negative zygote swims to a dark place and settles with the flagellar pole on a solid substratum. The nuclei of the two gametes are located at the flagellar poles. This
ensures that the nucleus of the zygote is at the pole selected for the formation of the rhizoid. The zygote starts to grow immediately.
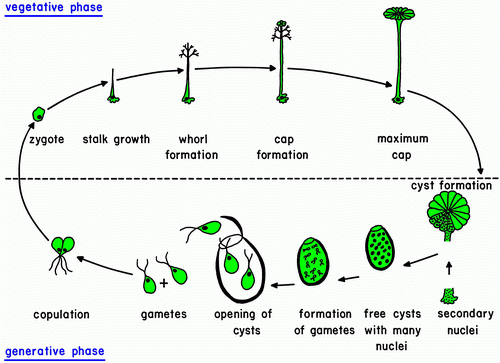
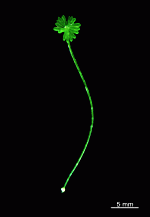
Example of a life cycle: Acetabularia sp.
Significance of Dasycladales to paleontologists and biologists
Paleontologists appreciate the calcification of the Dasycladales. Many of the periods during which large quantities of calcareous sediments were produced were also times when calcareous algae flourished. And many calcareous coats preserved morphology well
enough to enable paleontologists to follow the evolution of the Dasycladales through geologic time.
Existing Dasycladales live in littoral and sublittoral zones of warm-temperate and tropical oceans. As the environmental conditions of dasycladalean habitats are known, the preserved calcareous coats of fossils of these algae indicate the environment of the sediments of the large number of geological formations in which they have been found. Due to continental drift and changes of sea level dasycladalean fossils are found far from the present-day oceans, e.g. in the Alps and Carpathians. The fossils from these and other marine sediments now exposed contribute to the knowledge of paleoclimate and paleoecology (salinity, temperature, water depth, etc.). Some genera lived only during very restricted geological epochs, and consequently are used as indicator fossils for fine stratigraphic classification.
Contemporary Dasycladales attracted the attention of observers in ancient times. Pedianus Dioscorides, who served as surgeon in the army of the Roman emperor Nero (37-68 A.D.), wrote a treatise in Greek describing the properties of about 600 medical plants. Among these was Acetabularia from the Mediterranean Sea which he called Androsaces. His treatise was copied many times throughout the centuries and designated in Latin as De Medica Materia.
The species Acetabularia acetabulum was especially admired due to its fragile beauty. It was called by the fishermen "mermaid's wineglass". Species were collected and described by scientific globetrotters in the 16th, 17th, 18th and 19th century. However, their taxonomic classification and body plan were recognized only at the end of the 19th and in the first half of the 20th century.
The unique characteristics of the body plan and the reproductive methods of the Dasycladalean cells make them favourable subjects for biological research:
They
- are extremely large unicellular organisms (depending on the species 2 – 200 mm long)
- show a pronounced morphogenesis forming rhizoid (holdfasts), branched laterals (nutrition) and species-specific gametophores (reproduction)
- reveal a pronounced polarity
- tolerate extensive surgical manipulations, recover from and heal wounds in a short time
- have a high capability to regenerate
- allow transplantation of cell segments between different individuals of the same or different species
- possess only one cell nucleus (during the vegetative phase of their life cycle) which, taking into account the volume of the cell's cytoplasm, has an enormous size (up to 200 µm in diameter)
- have this nucleus always located in the rhizoid, so production of anucleate cells
is easy (by cutting off the rhizoid)
- allow easy manual isolation of the nucleus which may be kept alive outside the cell for more than 24 hours, microinjected or subjected to other treatments
- permit implantation of nuclei into anucleate fragments
- are easy to cultivate in the laboratory
Contribution of Dasycladales to knowledge of cell biology
It is to the credit of Joachim Hämmerling that Acetabularia flourished as a model subject for cell biological research. He succeeded in growing Acetabularia in laboratory cultures and discovered that through the entire vegetative phase of its life cycle this large unicell has only one nucleus and that this primary nucleus is always located in the rhizoid. Hämmerling made use of these features, as well as of the well-defined morphogenesis and of the high tolerance to surgery. By carrying out very clever and well-designed experiments he pioneered the knowledge in cell biology:
Long before the basis of the expression of genetic information was understood Hämmerling demonstrated that the nucleus is responsible for species-specific morphogenetic patterns. Based on the results which he obtained from growing cell fragments and anucleate cells, and by transplanting cell parts and implanting nuclei he postulated the existence of "morphogenetic substances". He showed that these substances are distributed with strict polarity.
Today we know that the morphogenetic substances are mRNPs (messenger RNA bound to proteins) and that their life-time is extended by protein binding. The polarity of Hämmerling's morphogenetic substances was eventually confirmed by the demonstration of a gradient of rRNA, mRNA and protein synthesis from the base to the apex of the cell. Morphogenetic substances have also been proven by the existence of a cap-specific mRNA. This RNA is produced early during growth of the cell but its translation is regulated by the cell nucleus which produces an inhibitor of cap formation for a certain time.
On the other hand, the cytoplasm too regulates activities of the nucleus. For example, only after the cap is mature will the nucleus undergo
meiosis, but removal of the cap will inhibit nuclear division. A combination of an "old" nucleus close to meiosis with young cytoplasm returns the nucleus to its early state so meiosis is deffered until the cell is mature. Vice versa a combination of a young nucleus with cytoplasm of a fully grown cell results in rapid meiosis.
Due to the open morphology of the Dasycladales it is easy to isolate chloroplasts devoid of any nuclear debris to study their autonomy. For example, it was shown for the first time in Acetabularia that while chloroplasts synthesize their own rRNA it is the nuclear genome that codes for their ribosomal proteins. This fact demonstrates an evolutionary loss of autonomy of these organelles. Another loss of chloroplast autonomy was demonstrated by considering Dasycladales as cell biological systems: the gene coding for dTMPkinase is located in the nucleus in Acetabularia while in Batophora it is located in the chloroplasts. In their evolutionary development the two genera separated 300 million years ago. An additional example of loss of organellar autonomy was given by a gene's involvement in the expression of circadian rhythmicity. Although this gene is located in the nucleus of higher plants, it is part of the chloroplast genome in the evolutionarily ancient Acetabularia.
Acetabularia also provided knowledge basic to the understanding of circadian rhythmicity. Anucleate cells keep their free-running circadian rhythm over several weeks. However, if a nucleus is implanted they change the phase of the rhythm to that of the mother cell of the nucleus. This observation together with other experiments making use of the implantation of the nucleus led to the detection that the nucleus initiates the circadian rhythm and determines its phase. However, the cytoplasm is capable of storing this information and regulating it on the level of translation. The observations of the circadian rhythm in Acetabularia led to the development of a "coupled translation membrane model". It postulates an "essential clock protein (ECP)". This protein was isolated from Acetabularia.
Acetabularia is also well suited for the study of cytoskeletal processes involved in the regulation of morphogenesis. And the fact that Acetabularia may be grown uncalcified in laboratory culture makes the cell transparent and allows investigations in cytoplasmic streaming which promotes the migration of chloroplasts and secondary nuclei. Elaborate morphogenetic models have been developed and tested experimentally in Acetabularia.
Thanks to Joachim Hämmerling and Hans-Georg Schweiger, who recognized the importance of an extensive collection of Dasycladales, other species became available for experiments. Twenty-six species were ultimately maintained in laboratory cultures under standard conditions of light and temperature.
They were originally housed in Germany at the Max-Planck-Institut für
Zellbiologie where they were used for research and sent to scientists working in various countries on the fundamentals of cell biology. Now they are included in the Culture Collection of Algae at the University of Texas, Austin, in the USA. From there they are available
to scientists all over the world:
Culture Collection of Algae (UTEX)
The University of Texas at Austin
MCDB Biology (A 6700)
205 W. 24th Street
Austin, TX 78712 - U.S.A.
http://www.zo.utexas.edu/research/utex/
Concluding remarks
As they are such favorite organisms in paleontological and cell biological research it is not surprising that an immense body of literature has accumulated covering the different fields of dasycladalean research. Three scientists who devoted many years to these fascinating organisms collected this literature and made the references available to other scientists interested in Dasycladales. Thousands of references on Dasycladales are listed on a CD:
Berger S., Granier B. & Bonotto S. (2004).- Dasycladales. Research
publications from the beginning until the year 2000.- 2 illustr., 373 p., CD-ROM, ISBN 3-906166-12-0, A.R.G. Gantner Verlag K.-G., FL 9491 Rugell (distributed by Koeltz Scientific Books, D 61462 Königstein)
During the time that I devoted my scientific life to research on Dasycladales, I collected a large number of photographs of these beauties. The selection of these photographs presented here is intended to provide information about the diverse species, make work with the Dasycladales easier and no less importantly to provide a means to enjoy their aesthetics.
Acknowledgments
The photographs were taken during my many years of research in the Max-Planck-Institut für Zellbiologie in Wilhelmshaven and later in Ladenburg (both in Germany). I highly appreciate the theoretical and practical help of everyone who had a share in my work with the Dasycladales.
I am indebted to Larry Liddle and Nestor Sander for critical reading of the English manuscript. My particular thanks go to Bruno Granier, whose highly
motivated participation made the publication of these photographs first possible.
Selected publications on Dasycladales
Berger S. & Kaever M.J. (1992).- Dasycladales: An Illustrated Monograph of a Fascinating Algal Order.- Georg Thieme Verlag, Stuttgart, New York: 247
p.
Berger S., de Groot E.J., Neuhaus G. & Schweiger M. (1987).- Acetabularia: a giant single cell organism with valuable advantages for cell biology.- European Journal of Cell Biology 44: 349-370.
Berger S., Fettweiss U., Gleissberg S., Liddle L.B., Richter U. & Sawitzky H. (2003).- 18S rDNA phylogeny and evolution of cap development in Polyphysaceae (formerly Acetabulariaceae; Dasycladales, Chlorophyta).- Phycologia 5: 506-561.
Bonotto S. (1998).- Recent progress in research on Acetabularia and related Dasycladales.- Progress in Phycological Research 6: 59-235.
De Castro P. (1997).- Introduzione allo studio in sezione sottile delle Dasicladali fossili An approach to thin-section study of fossil Dasycladales.- Quaderni dell'accademia pontaniana 22: 261
p.
Deloffre R. & Granier B. (1993).- Inventaire des Algues Dasycladales fossiles. I° partie - Les Algues Dasycladales du Tertiaire.- Revue de Paléobiologie 11/2 (1992): 331-356.
Graham L.E. & Wilcox L.W. (2000).- Algae.- Prentice Hall, Upper Saddle River, NJ, USA: 640
p.
Granier B. & Deloffre R. (1994).- Inventaire des Algues Dasycladales fossiles. II° partie - Les Algues Dasycladales du Jurassique et du Crétacé.- Revue de Paléobiologie 12/1 (1993): 19-65.
Granier B. & Grgasović T. (2000). - Les Algues Dasycladales du Permien et du Trias. Nouvelle tentative d'inventaire bibliographique, géographique et stratigraphique.- Geologia Croatica 53/1: 1-197.
Hämmerling J. (1963).- Nucleo-cytoplasmic interactions in Acetabularia and other cells.- Annual Review of Plant Physiology 14: p. 65-92.
Mandoli D.F. (1998).- Elaboration of body plan and phase change during development of Acetabularia: How is the complex architecture of a giant unicell built?- Annual Review of Plant Physiology and Plant molecular Biology 49: 173-198
Menzel D., Jonitz H. & Elsner-Menzel C. (1992).- The cytoskeleton in the life cycle of Acetabularia and other related species of dasyclad green algae.- In: Menzel D. (ed.), The Cytoskeleton of the Algae.- CRC Press, Boca Raton: 195-217.
Pia J. von (1912).- Neue Studien über die triadischen Siphoneae verticillatae.- Beiträge zur Paläontologie und Geologie Österreich-Ungarns und des Orients XXV: 25-81, pls. II-VIII [I-VII]
Pia J. von (1920).- Die Siphoneae verticillatae vom Karbon bis zur Kreide. Abhandlungen der zoologisch-botanischen Gesellschaft in Wien XI/2: 259
p., pls. I-VIII [Les Siphonées verticillées du Carbonifère au Crétacé (1961).- Éditions Technip, Rueil-Malmaison: 258
p., pls. 1-8].
Schweiger H.G. & Berger S. (1979).- Nucleocytoplasmic interrelationships in Acetabularia and some other Dasycladaceae.- International Review of Cytology, Suppl. 9: 11-44.
Valet G. (1968).- Contribution à l'étude des Dasycladales. 1. Morphogenèse.- Nova Hedwigia XVI: 21-82, pls. 4-26.
Valet G. (1969).- Contribution à l'étude des Dasycladales. 2. Cytologie et reproduction. 3. Révision sytématique.- Nova Hedwigia XVII: 551-644, pls. 133-162.
van den Hoek C., Jahns H.M. & Mann D.G. (1993).- Algen.- Georg Thieme Verlag, Stuttgart, New York, 3. Auflage: 411
p.
Photo-Atlas
Dasycladaceae
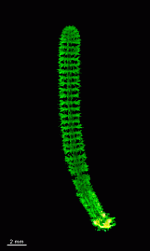
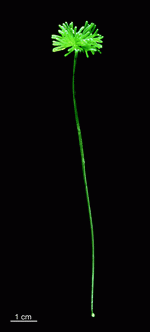
Batophora
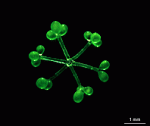
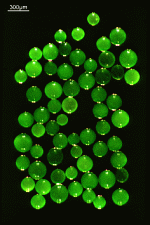
B. occidentalis
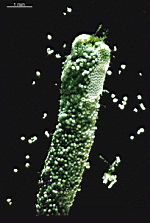
- upper part of a gametophore-bearing cell
- isolated whorls with gametophores
- isolated whorl with gametophores
B. occidentalis var. largoensis
- part of a field-collected cell with gametangia-bearing gametophores
- part of a field-collected cell with gametangia-bearing gametophores
- part of a field-collected cell with gametangia-bearing gametophores
- upper part of a cell grown in the laboratory, f1 generation. The gametophores bear gametangia
- upper part of a cell grown in the laboratory, f2 generation
- isolated whorl with gametangia-bearing gametophores
- gametangia shortly before release of gametes
- chloroplasts of B. oerstedi (upper micrograph) and B. occidentalis var. lagoensis
B. oerstedi
- young cell
- young cell
- young cell
- young cell
- young cell with condensed cytoplasm around the nucleus
- young cell with condensed cytoplasm around the nucleus
- beginning of gametophore formation
- beginning of gametophore formation
- beginning of gametophore formation
- beginning of gametophore formation
- upper part of a cell with gametophores
- upper part of a cell with gametophores
- upper part of a cell with gametophores
- part of a cell with gametophores
- part of a cell with gametophores
- part of a cell with gametangia-bearing gametophores
- one whorl with gametangia-bearing gametophores
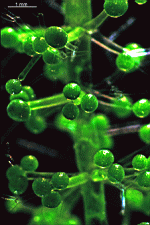
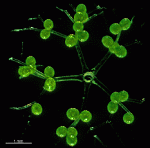
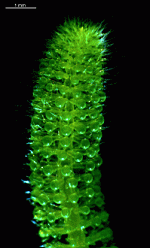
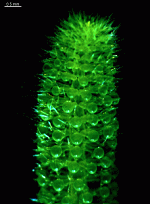
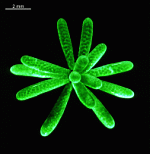
Bornetella
B. oligospora
- young cell
- two young cells with pseudocortex
- young cell with pseudocortex
- young cell with pseudocortex
- immature cell
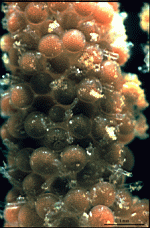
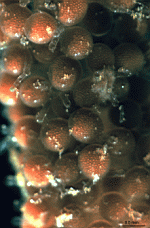
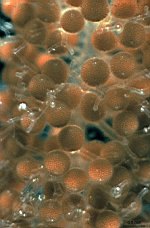
B. sphaerica
- a cluster of young cells
- a cluster of young cells
- young cells
- young cells
- young cells
- two young cells just after cortex formation
- growing cell
- growing cell
- cell
- cell
- cell
- faceted cortex of a cell shortly before formation of gametophores
- enlarged faceted cortex of a cell shortly before formation of gametophores
- a cluster of young and mature cells
- two grown cells in a cluster of young cells
- a cluster of young and mature cells
- three cells with gametophores
- cell with gametophores
- cell with gametophores
- longitudinal section through a cell with gametophores
- longitudinal section through a cell with gametophores
- cross section through a cell with gametophores
Chlorocladus
Chlorocladus australasicus
- young cell
- upper part of a cell with gametophores
- upper part of a cell with gametophores
- upper part of a cell with gametophores
- part of a cell with cyst-bearing gametophores
- part of a cell with cyst-bearing gametophores
- part of a cell with cyst-bearing gametophores
- one whorl with gametophores containing gametangia
Cymopolia
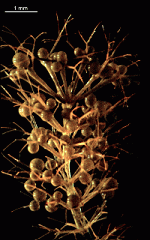
C. barbata
- upper part of a cell
- upper part of a cell
- upper parts of two cells
- upper parts of two cells
- young cells growing out of a mature cell
- Cymopolia barbata in its natural habitat (by courtesy of Larry B. Liddle, Long Island University, New York, USA)
- Cymopolia barbata in its natural habitat (by courtesy of Larry B. Liddle, Long Island University, New York, USA)
C. vanbosseae
- cell
- cell
- cross section
- longitudinal section
Dasycladus
D. vermicularis
- young cell
- upper part of a young cell
- beginning of gametangia formation
- beginning of gametangia formation
- part of a mature cell
- part of a mature cell
- most gametangia have released gametes
- gametangia close to release of gametes
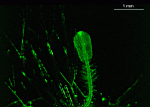
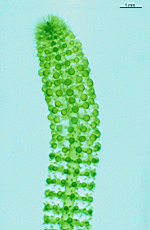
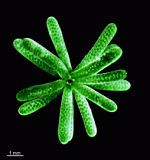
Neomeris
N. annulata
- mature cell grown in laboratory culture
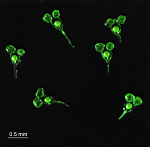
- five mature cells as they appear in their natural habitat
- mature cells collected in their natural habitat
- gametophores made coherent by thick calcareous encrustation
- Neomeris annulata in its natural habitat (by courtesy of Ikuko Shihira-Ishikawa, Institute of Physical and Chemical Research (RIKEN), Japan)
N. dumetosa
- young cells
- young cells, one with pseudocortex
- young cell with pseudocortex
- three cells
- upper part of a cell
- upper part of a cell
- mature cell as it appears in its natural habitat
- mature cell as it appears in its natural habitat
- upper part of a mature cell as it appears in its natural habitat
- upper part of a cell collected in its natural habitat
- isolated laterals with gametophores
Polyphysaceae
Acetabularia
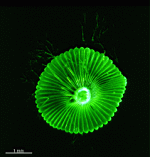
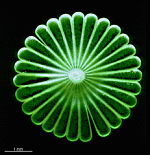
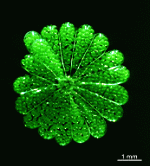
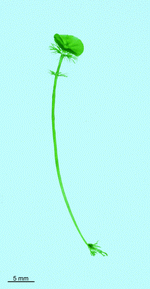
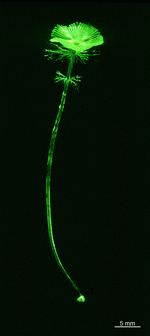
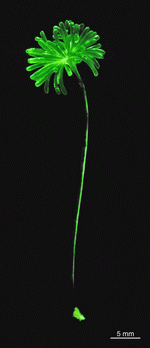
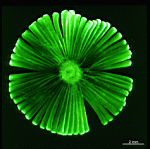
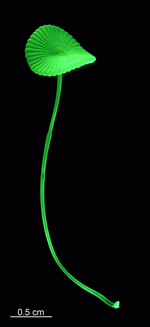
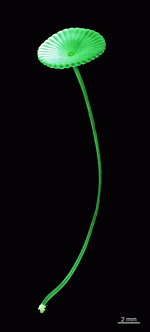
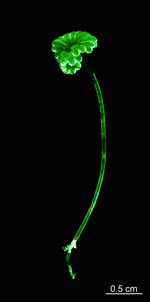
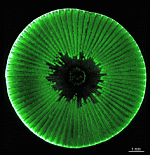
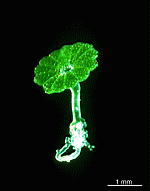
A. acetabulum
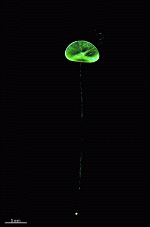
- cell
- cell
- cell
- three fully grown cells
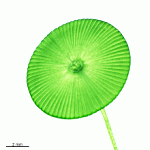
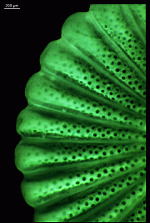
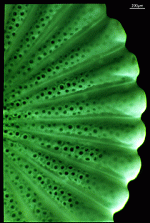
- fully grown cap
- fully grown cap
- upper part of a fully grown cell
- upper part of a fully grown cell
- cell with gametangia (cysts)
- upper part of a cell with gametangia
- cap with gametangia
- cap with gametangia
- cap with gametangia
- field-collected rock with A. acetabulum
- upper part of a cell growing in its natural habitat
- upper parts of two cells growing in their natural habitat
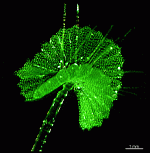
- cap of a cell growing in its natural habitat
- cap of a cell growing in its natural habitat
- cap of a cell growing in its natural habitat
A. caliculus
- young cell with persistent whorls
- cell with a young cap
- cell with a young cap
- cell with a young cap
- upper part of a cell with a young cap
- upper part of a cell with a gametangia (cyst)-bearing cap
- mature cell opening to release gametangia
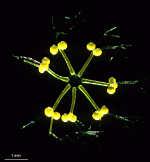
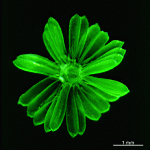
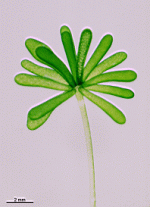
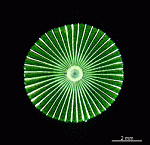
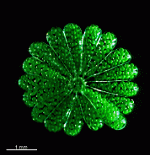
A. crenulata
- cell
- cap
- cell
- cell
- cell
- cell
- cell
- cell
- young cap
- young cap
- cap
- cap
- cap of a cell as it appears in its natural habitat, seen from below
- cap of a cell as it appears in its natural habitat
- cap rays of a cell as it appears in its natural habitat
- cap rays of a cell as it appears in its natural habitat
- cap rays of a cell as it appears in its natural habitat
- cap rays of a cell as it appears in its natural habitat
- cap rays of a cell as it appears in its natural habitat
- cap rays of a cell as it appears in its natural habitat. Rays open at their tips to release gametangia (cysts)
- part of a gametangia-bearing cap collected in the natural habitat
A. dentata
- A. dentata growing in its natural habitat (by courtesy of Ikuko Shihira-Ishikawa, Institute of Physical and Chemical Research (RIKEN),
Japan)
- A. dentata (right) and P. parvula in their natural habitat
- cap of a cell in its natural habitat
- upper part of a cell in its natural habitat
- cap of a cell as it appears in its natural habitat
- cap of a cell collected in its natural habitat seen from below
- cell with gametangia (cysts)
- cap
- cap
A. farlowii
- one cell
- cap with gametangia (cysts) seen in the natural habitat
- cap with gametangia as it appears in the natural habitat
- several calcified caps as they appear in the natural habitat
- young cap
- upper part of a cell with gametangia
- cap with gametangia
A. kilneri
- cell with very young cap
- cell with immature cap
- cell with immature cap
- cell with not fully grown cap
- cell
- cell
- cell
- cell
- cap with gametangia (cysts)
A. major
- one cell
- upper part of a cell
- cap from above
A. peniculus
- cell
- cell with gametangia (cysts)
- upper part of a cell
- upper part of a cell starting to form gametangia
- upper part of a cell
- upper part of a cell with gametangia
- cap with gametangia
- cap with gametangia
- isolated gametangia (cysts)
A. ryukyuensis
- A. ryukyuensis in its natural habitat (by H.G. Schweiger, Max-Planck-Institut, Ladenburg, Germany)
- A. ryukyuensis in its natural habitat (by H.G. Schweiger, Max-Planck-Institut, Ladenburg, Germany)
- A. ryukyuensis in its natural habitat (by H.G. Schweiger, Max-Planck-Institut, Ladenburg, Germany)
- A. ryukyuensis in its natural habitat (by H.G. Schweiger, Max-Planck-Institut, Ladenburg, Germany)
- cells on a rock collected in their natural habitat (by courtesy of Ikuko Shihira-Ishikawa, Institute of Physical and Chemical Research (RIKEN),
Japan)
- A. ryukyuensis and Halicoryne spec. growing in their natural habitat
(by courtesy of Ikuko Shihira-Ishikawa, Institute of Physical and Chemical Research (RIKEN),
Japan)
- cell grown in laboratory culture
- cell grown in laboratory culture
- upper part of a cell grown in laboratory culture
- cap of a cell grown in laboratory culture
A. schenckii
- cell
- cell
- cell
- upper part of a cell
- upper part of a cell
- upper part of a cell
- upper part of a cell with gametangia (cysts)
- cap of a cell grown in laboratory culture
- cap with gametangia (cysts) of a cell grown in laboratory culture
- cap revealing the calcification of gametangia
- cap revealing the calcification of gametangia
- cap revealing the calcification of gametangia
- part of a cap revealing the calcification of gametangia seen from below
- part of a cap revealing the calcification of gametangia
- part of a cap revealing the calcification of gametangia
Chalmasia
C. antillana
- cell
- upper part of a cell
- upper part of a cell with gametangia (cysts)
- upper part of a cell with gametangia
- cap with gametangia
- part of a gametangia-bearing cap grown in laboratory culture, partially calcified, especially
the lids of the gametangia
- gametangia-bearing cap as it appears in its natural habitat
- gametangia-bearing cap as it appears in its natural habitat
- gametangia-bearing cap as it appears in its natural habitat
- gametangia-bearing cap as it appears in its natural habitat
- gametangia-bearing cap as it appears in its natural habitat
- gametangia-bearing cap collected in its natural habitat
- gametangia-bearing cap collected in its natural habitat
Halicoryne
H. spicata
- young cell
- young cell
- upper part of a young cell
- upper part of a cell, in part with gametangia
- upper part of a cell with gametangia (cysts) growing in its natural habitat
- upper parts of two cells growing in their natural habitat
- upper parts of two cells, in part with gametangia, grown in their natural habitat
- upper part of a cell with gametangia growing in its natural habitat
- part of a cell with lime-spicule embedded gametangia growing in its natural habitat
- lime spicules collected in the field
- upper part of a cell, SEM micrograph
Parvocaulis
P. clavata
- a cluster of entangled cells
- a cluster of entangled cells
- a cluster of very young and cap-bearing cells
- cell
- three cells
- cap prior to gametangia (cyst) formation
- cap prior to gametangia formation
- two gametangia-bearing caps
- gametangia-bearing cap
P. exigua
- cells in several stages of development, growing on a rock
- cells in several stages of development, growing on a rock
- several cells, growing on a rock
- several cells growing on a rock
- cap of a cell, growing on a rock
- caps of cells, growing on a rock
- cap with gametangia (cysts), growing on a rock
- cell with gametangia in cap and stalk, growing on a rock
- cell with immature cap
- cap with gametangia
P. parvula
- cell
- cell
- cell
- cell
- cell with gametangia (cysts)
- cell with gametangia
- cell with gametangia
- cell with gametangia
- four cells in different stages of development
- P. parvula growing on a rock
- P. parvula growing on a rock
P. polyphysoides
- cap with gametangia (cysts) and very young cap in a cluster of entangled young cells
- cap
- cap with gametangia
- cap with gametangia
- immature cap of a cell growing in its natural habitat
P. pusilla
- a cluster of entangled cells, three of which are cells with gametangia (cyst)-bearing caps
- cap with gametangia
- several cells
- several cells
- cell with cap
- three cells, two with gametangia
- cap
- cap
- cap with gametangia
- cap with gametangia
- cap with gametangia
Velum of Parvocaulis
- velum of P. pusilla
- velum of P. pusilla
- velum of P. polyphysoides
- velum of P. polyphysoides
- velum of P. clavata
- velum of P. clavata
- velum of P. clavata
- velum of P. clavata
- velum of P. exigua
- velum of P. exigua
- velum of P. exigua
Example of a life cycle: the Polyphysaceae
- many germinating zygotes, about 10 days old. A. acetabulum
- growing zygote, nucleus visible. A. acetabulum
- rhizoid starts to form, nucleus still visible. A. acetabulum
- young cell before whorl formation (20 days old). A. acetabulum
- young cell before whorl formation. A. acetabulum
- growing tip. A. acetabulum
- whorl formation stage 1. A. acetabulum
- whorl formation stage 2. A. acetabulum
- whorl formation stage 3. A. acetabulum
- whorl formation stage 4. A. acetabulum
- whorl formation stage 5. A. acetabulum
- growth of a new tip after whorl formation. A. acetabulum
- growth of a new tip after whorl formation. A. acetabulum
- whorl formation stage 1. A. acetabulum
- whorl formation stage 2. A. acetabulum
- whorl formation stage 3. A. acetabulum
- whorl formation stage 4. A. acetabulum
- growth of a new tip after whorl formation. A. acetabulum
- upper part of a cell with several sets of whorls. A. caliculus
- cell with whorls. A. acetabulum
- lower part of a cell with rhizoid. A. acetabulum
- two rhizoids. A. acetabulum
- nucleus of a cell approaching cap formation (nucleus is compressed into the stalk,
so it is visible). A. acetabulum
- cell with young cap. A. kilneri
- young cap. A. crenulata
- young cap. A. schenckii
- fully grown cap. A. acetabulum
- several stages of gametangia (cyst) formation in one cap. A. acetabulum
- cap with gametangia. A. acetabulum
- cap with gametangia. A. acetabulum
- cap with gametangia. A. farlowii
- cap with gametangia. A. peniculus
- gametangia-bearing cell. P. parvula
- cap with gametangia. P. exigua
- opening of cap rays to release gametangia. A. crenulata (in natural habitat)
- opening of cap rays to release gametangia. A. caliculus
- gametangia. P. parvula
- gametangia. P. parvula, left, and A. acetabulum, right
- release of gametes. A. acetabulum
- release of gametes. P. parvula
- release of gametes. A. acetabulum
- release of gametes. A. acetabulum
- several stages of release of gametes. A. acetabulum
- gametes. A. acetabulum