Short Treatise on Foraminiferology
(Essential on modern and fossil Foraminifera)
_
Jean-Pierre Bellier
Robert Mathieu
Bruno Granier
Foreword
After some forty years in the academic world and before abandoning a position as an employee of the State educational system to enter the new world of "pensioners", it seemed worthwhile to the first author
(J.-P.B.) of this brief discussion to compile a short document that presents a synopsis of the knowledge acquired, taught and put to use for more than four decades. This fascicle reviews only the foraminifers, which, along with calcareous nannofossils, are the fundamental tools of
modern biostratigraphy, used for worldwide oceanographic studies and for the correlation of oil wells. It
deals essentially with the
small foraminifers, that is those of modest size without a complex internal architecture. These include the planktonic foraminifers of which the rapid evolution and great dispersion permit the establishment of reliable biochronologic scales of reference.
This short course is intended for students preparing for the CAPES (Certificat d'aptitude au professorat de l'enseignement du second degré
= Certificate of Aptitude for a Professorship of Instruction in the Second Grade), for students working toward the Aggregation in Natural Sciences in pursuit of a scientific career in the universities or in the
CNRS (Centre National de la Recherche Scientifique = National Centre for
Scientific Research), and for students who hope to work in the oil industry where a specialization in the field of micropaleontology
is considered useful and is still
valued. It will also interest amateur naturalists and that portion of the general public attracted by the beauties of the microscopic world, now living, or extinct and represented only by
fossils.
Introduction
The foraminifers are unicellular organisms of the animal kingdom that live on the bottom of lagoons and seas, or else are a part of the oceanic plankton. The cytoplasm of the cell of these protozoans is for the most part covered by a shell
(test) which may be composed of organic matter, mineral substances, or agglutinated particles. The test has one or more chambers which may have one or several apertures,
foramina (a term from which the name of the group is derived), that allow the chambers to communicate with each other
or with the exterior. The cytoplasm emerges from these outer orifices, covers more or less the test, and emits fine and reticulated
pseudopodia with which the microorganism fixes itself on the substratum, moves, and captures its prey. Foraminifers are recognized from the beginning of the
Paleozoic Era to the Present. Certain of their lineages evolved rapidly which is useful for
biochronology and for precise interregional correlation of geologic strata. Their ecologic sensitivity makes them especially useful in the study of existing and ancient
environments.
The following text and figures are but an outline of this fundamental branch of micropaleontology. Emphasis will be focused mainly on the
"small foraminifers" that is those in which the larger dimensions of the tests are about half a millimeter and in which the chambers lack complex internal constructions or architecture. Their
systematics and evolutionary relationships will be touched on only in broad outline. The foraminifers record variations in the isotopes of 13C
and 18O but this geochemical aspect of their use is not within the domain of fundamental micropaleontology and is not discussed. Planktonic foraminifers will be accorded special attention, given their outstanding value for scientific deep sea
drilling programs and in oil well biosteering.
I. General characteristics
The foraminifers are Protozoa with a shell (= test) that consists of successive chambers that intercommunicate through orifices called
foramina. The chambers are separated from each other by partitions called septa. The last chamber communicates with the exterior through one or several apertures. Cytoplasm that completely fills all the chambers emerges through these exterior apertures and covers the outside of the test where it emits
fine filamentous granular and reticulate pseudopodia; these often include grains or fine particles of various kinds and play an important role in maintaining the living organism: movement, food supply, construction of new chambers,
etc. Symbiotic algae (dinoflagellates) are often present in the cytoplasm.
The life cycle of the Foraminifera involves an alternation of generations. Their size generally ranges between 0.1 and 1 mm; some can attain 10 cm or
more. For a one-celled organism these are truly gigantic!
Foraminifers are found in all marine environments. Some are adapted to brackish
water. There are none in fresh water. Two kinds are recognized:
- Benthic (Figs. 1
 - 2
- 2  ). These are forms that live on the sea
floor, either on the surface of the sediment or buried in it (endofauna), or are attached to plant stems, rocks or particles
(epifauna);
). These are forms that live on the sea
floor, either on the surface of the sediment or buried in it (endofauna), or are attached to plant stems, rocks or particles
(epifauna);
- Planktonic (Fig. 3
 ).
These are forms which float passively, moved only by currents but capable of vertical migration.
).
These are forms which float passively, moved only by currents but capable of vertical migration.
Foraminifers with tests of organic matter exclusively are not
thoroughly understood [0rder Allogromiida]. They are mostly unilocular forms that have been studied only rarely by
micropaleontologists, because their remains do not preserve easily and so are
rarely represented in fossil assemblages. The tests of most of the
foraminifers we study are biomineralized.
Three types of wall are recognized in living foraminifers with calcitic
tests:
- Agglutinated: the wall has a composite appearance. It consists of
foreign
agglutinated particles (quartz grains, sponge spicules, Nannoconus,
etc.) imbedded in a chitinous or
calcareous cement secreted by the organism [orders Astrorhizida, Lituolida, Trochamminida and Textulariida].
Remark: Beginning in Cretaceous times some rare forms seem to have a siliceous
(?)
test [Order Silicoloculinida] among which is
the genus Rzehakina Cushman, 1927;
- Porcellaneous: the calcareous wall, secreted entirely by the
animal, has a uniform aspect. It is imperforate, smooth and homogeneous.
The surface is a brilliant white or amber resembling porcelain [Order Miliolida];
- Hyaline: the wall, again calcareous and secreted by the animal, is
perforate. If there are ornaments, they have the appearance of the remainder of the
wall: vitreous, transparent or translucent [orders Carterinida (test of macrocrystals), Spirillinida
(with a pseudo-monocrystalline fabric), Lagenida,
Buliminida, Rotaliida
and Globigerinida]. Remark: Starting in the Permian there
are offshoot
forms with tests that were originally aragonitic, not calcitic [orders Involutinida
and Robertinida].
These three types of calcitic test exist as fossils and in addition a fourth
type has been identified. Some Paleozoic foraminifers
have a calcareous test with a texture called microgranular [0rder
Fusulinida], which like that of the
porcellanids and hyalinids is composed of microcrystalline calcite, but its crystals are often so arranged as to give a striated aspect to
thin sections (so-called a pseudofibrous
wall).
The unilocular test, the simplest form, consists of a single spherical or tubular
chamber. Much more common is a test of several chambers. A chamber is the unit
added to the previous test during a growth step, i.e. an instar. It may
be divided into subunits called chamberlets; they all grow at the same instar.
Chambers increase in size and are separated by septa. The line marking the
junction between two successive chambers, sometimes visible on the outer surface
of the test, is called a suture. The number of chambers and their form and
arrangement are extremely varied. Consequently the general aspects of
multilocular tests are exceedingly diverse.
1) In the rectilinear or curved mode the chambers are placed in a straight or
slightly curved
alignment. If there is but one row of chambers the test is uniserial. If there are several
rows, it is bi-, tri-, or multi- serial.
2) In the enrolled mode the chambers are arranged in a spiral. If the spiral is plane the test
is planispiral, with two identical faces. When all the whorls of the spire are
visible on either side enrollment is evolute. Coiling is involute when the final whorl covers all of the preceding
ones. The umbilicus is located in the central portion of the spire's outer
surface where the sutures of a whorl join each other.
If the spire is trochoid the two faces of the trochospiral test differ. One of
them, evolute, shows the coils of the spire, the other, called the umbilical side, is involute.
Remarks:
- The direction of enrollment is not always uniform in all the individuals of any one species
(dextral or sinistral) for it is dependent on variations in climate or
environment.
- In most cases the enrolled chambers are uniserial. Very exceptionally they are
biserial.
3) In annular or cyclical tests the chamberlets are arranged in concentric rings.
4) The miliolids have a special arrangement of chambers called milioline. The curved
chambers, tangent at their two extremities to the axis of prolongation, are arranged in
quinque-, tri-, or bi- loculine cycles.
5) Along with these simple constructions, numerous tests have several
successive but discrete modes of growth. This is called mixed or composite
development.
The
distributional pattern of the apertures is of great importance for the function
of the shell and for its placement in the taxonomic system. In benthic forms,
the last chamber has a frontal wall, the apertural face, on which a single or
multiple apertures provide communication of the last chamber with the
exterior. Apertures are extremely variable in both form and placement.
The main aperture may be " simple" that is round, a slit, crescent-shaped, radial or dendritic, sometimes at the top of a collar bordered by a smooth or crenulate lip, or partially covered over by calcareous
structures such as a tooth, bulla,
etc.. On the other hand it may be "multiple" with several small orifices in a line or scattered.
Its position is variable: basal, terminal, sutural, or
peripheral. In a trochospiral test it is median, umbilical or spiral.
In forms that live in an
isotropic environment, planktonic forms in particular, additional apertures may
be distributed in the septal sutures. Benthic smaller
foraminifera exhibit a peculiar structure attached to the inner side of the aperture, a
so-called tooth plate. Its function is not yet understood, but taxonomically its
type and location are very important.
The ornamentation involves:
- The surface of the test, smooth or covered with striae, ridges, a
reticulation, tubercles, spines,
etc.;
- The sutures, plain or limbate, which means accentuated by a ridge of
variable width;
- The periphery of the test may or may not be marked by spines or a single or double
keel;
- The umbilicus may be open or occupied by a
simple or composite plug.
The great number of combinations possible among these test walls,
chamber conformations and wide range of ornamentations has led to the extreme morphologic diversity of the
foraminifers.
Stratigraphically, the foraminifers appear in the Early Cambrian (the first unambiguous ones are from the
lowermost Cambrian of West Africa) at about the same time that the metazoans with
skeletons present themselves. Molecular studies indicate this appearance must have been preceded by a broad radiation of
naked, practically unfossilizable unicellular species that diverged from a
Cercozoarian ancestor several million years before the beginning of the Phanerozoic
(Pawlowski et alii,
2003). The tubular microfossil Platysolenites described
from the uppermost Precambrian of several regions has not been assigned definitively either to worms or
to the foraminifers (Langer,
1999). Planktonic foraminifers appeared in the Toarcian
(Early Jurassic). The possible existence of "planktonic" taxa in the Trias-Jurassic
strata of the offshore of northwestern Australia has not yet been resolved
(Hart et alii,
2007).
The foraminifers were particularly affected by the great crises: for example the
Permo-Triassic extinction,
and the major changes at the Cretaceous-Paleogene
transition. Their study brought to light the catastrophic nature of certain major transitions,
also evinced in a number of other groups of organisms. These abrupt transitions also represent important cutoffs in geologic time. On a smaller scale the recognition of the succession of species in well-documented families
of foraminifers provides a tool for dating that can be
extremely
precise. In
addition, their sensitivity to living conditions makes them very useful as indicators of (paleo-)
environment.
Historically, foraminifers have been known since Antiquity: Herodotus
(around 484-425 B.C.), Strabo (63 B.C. to 20 A.D.)
and Pliny the elder (23-79 A.D.) mentioned an
accumulation of lens-shaped objects in the limestones of the Egyptian pyramids:
they had detected the presence of Nummulites (large benthic foraminifers). The
Class Foraminifera was created at the beginning of the 19th century by Alcide d'Orbigny
(1802-1857) in his work entitled "Tableau méthodique de la
classe des Céphalopodes"
(1826). Founder of Micropaleontology, d'Orbigny
described more than 1000
species and was the first to study the way of life and the ecology of these
small organisms that he classed with the ammonites. The unicellular nature of
the foraminifers was discovered in 1835 by Félix Dujardin
(1801-1860). The samples from oceanic deeps recovered by the first scientific
oceanographic survey of the "Challenger" (1873-1876), studied until
the 1880's by specialists like Brady, produced a
quantity of fundamental data on both living and fossil forms. The work done in
the 20th century developed the use of foraminifers as biostratigraphic tools.
Associated with this work are prestigious names such as Cushman, Loeblich
and Tappan in the United States, Subbotina
in the Soviet Union, Bolli and Sigal
in Europe, just to list few of them.
II. Some information on living forms
1. Food
Benthic foraminifera that are active herbivores graze on algae and bacteria while moving on their
substrate. Passive herbivores are sessile epifaunal forms that capture their food
(diatoms) around the site where they are fixed. The prey of the carnivores are small arthropods and other foraminifera.
Their digestion takes nearly a
day. Some are suspension feeders. The majority of the foraminifera living in the fine sediment under the photic zone eat detritus and
bacteria. They predominate in the uppermost levels (1-2 cm) just under the
sediment-water interface. Most benthic foraminifers are opportunistic omnivores
but many large forms have symbiotic algae that
aid in supplying energy.
Spinose species of plankton eat for the most part zooplankton like copepods. Their needs are estimated at a capture followed by digestion every 24
hours. They also consume other types of
crustaceans, tintinnidae, radiolaria, polychaeta and the larvae of gastropods, pteropods and
tunicates. Calanoid copepods are digested in 7 to 9 hours, cyclopoid copepods take longer, 9 to 20
hours. Harpacticoid copepods are eaten only exceptionally. The species without spines eat mainly phytoplankton: mostly diatoms, along with coccolithophorids and
dinoflagellates. This type of foraminifer may ingest copepods but only when they have been immobilized or are
dead. Digestion times are much longer than those observed in spinose
species (according to Hembelen et alii,
1989).
When swallowed by others organisms (worms, gastropods, crustaceans, echinoderms, fish,
...) foraminifers commonly pass through their digestive systems without being
assimilated.
2. Functions of the test
The test has six possible functions (after Murray,
1991):
- to protect the animal from predation;
- to act as a barrier against an unfavorable environment;
- to be used as a receptacle for excreted matter;
- to aid in the process of reproduction;
- to control the movement of the organism;
- to help in the growth of the cell.
Hyaline foraminifers have more porous tests in warm and
less saline waters (Hembelen et alii,
1989). These waters are also less dense so the porosity
of the tests of planktonic foraminifers can be used as a paleoceanographic tool to evaluate the
temperature, salinity and relative density of sea water. The dimensions and
density of "perforations" are all criteria for systematic distinctions in both living and fossil planktonic
foraminifers: for example, macro- and micro- perforate forms
are set apart in the classification of Early Cretaceous genera
(Moullade et alii,
2002).
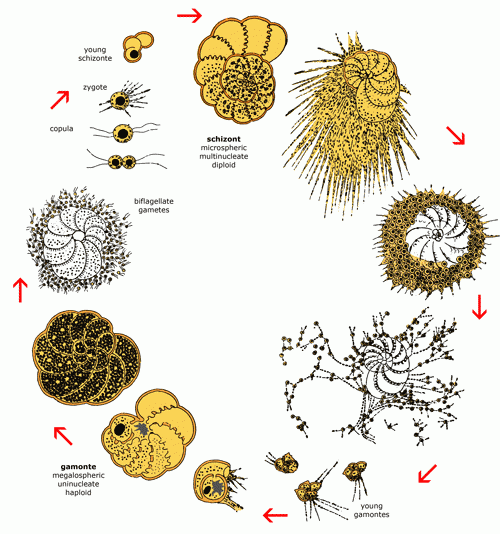
3. Reproduction
Of the approximately 4,000 living species, the life cycle of only 30 of them is
well-known. Studied in the laboratory the
cycle of benthic foraminifers is haplo-diplophasic. One generation is haploid, the
next one diploid
(Fig. 4  ). The haploid forms are called
gamonts. They, by division of the nucleus, produce gametes (undifferentiated sex
cells). The fusion of two gametes produces a diploid
individual, the schizont, which is
multinucleate and reproduces through mitosis. After meiosis and division of the cytoplasm around each nucleus, the schizonts produce new
gamonts. Gamonts and schizonts are distinguishable both by test size and by the dimensions of their initial
chamber. The gamonts are also called the megalospheric form and the schizonts the microspheric
form.
). The haploid forms are called
gamonts. They, by division of the nucleus, produce gametes (undifferentiated sex
cells). The fusion of two gametes produces a diploid
individual, the schizont, which is
multinucleate and reproduces through mitosis. After meiosis and division of the cytoplasm around each nucleus, the schizonts produce new
gamonts. Gamonts and schizonts are distinguishable both by test size and by the dimensions of their initial
chamber. The gamonts are also called the megalospheric form and the schizonts the microspheric
form.
In planktonic foraminifers, there is no dimorphism and probably no asexual reproduction in accordance with
their strategy of life. In many species a layer of calcite known as the "calcitic crust"
is secreted on the chambers of the last whorl during
gametogenesis; its function is
unknown (resistance to dissolution?).
A great number of fossil forms were described before the life
cycle of the foraminifers was
known. A number of morphotypes differing only in size and in the dimensions of the
initial chamber
(the proloculus) were called discrete species. Readjustments after the discovery of the
two phases of the cycle led some micropaleontologists to give a double specific name
to one taxon, a practice that is contrary to the rules of Linnean nomenclature.
Among the Nummulites such double
specific names are particularly frequent.
4. Productivity
The longevity of foraminifers is difficult to evaluate.
It ranges between 15 days and 16 months. The tests of numerous foraminifers are
partially dissolved or disintegrated during the reproductive phase. On
the substratum the production of carbonate by large benthic
tropical or subtropical foraminifera has been measured as ranging from 40 to 2,800 grams per m2
a year. Counts indicate that the density of populations on the sea floor
varies from 0 to 8,600 foraminifers per 10 cm2. The tests of small,
thin-walled forms are often transported in suspension; these are the adults of
small species and the immature forms of of larger species, both generally less
than 200 µm in diameter or length (Murray,
1991). In many cases they are transported post mortem
far from their source, often for more than 100 km.
The great geologic interest concerning planktonic
foraminifers is a function of their immense productivity. They live in the whole
water column. The greatest concentrations are found at depths of 10 to 50 meters. Living populations range in number from from 1 to 200 per m2
near the surface of the oceans. Today both individuals and species are more abundant in warmer
waters. Up to 10,000 foraminifers per m3
of sea water have been counted, but at
depths of more than 1000 m the count is less than 1 in 100 m3. It is
estimated that today 47% of the ocean bottom, or about 126,000,000 km2 are
covered by their shells that fall in a constant rain at a mean velocity of about 2
cm/sec to the sea floor. Several thousand specimens may be contained in a gram
of sediment.
III. Methods of study
For examination of their foraminiferal content soft sediments (sands, clays, marls, etc.) are washed in tap water
passing through very fine-meshed
(usually
63 µm) sieves. The operation may be facilitated by stirring the sample with a soft
brush. It is completed when the water runs
clear. The residues
(washed samples) are dried and graded using dry screens. They are then sorted under a binocular microscope with a magnification of between 20X and
100X using a needle coated with plastiline or better a very fine (000) damp
brush. The sorting selects and segregates the tests in cells on cardboard slides
while insofar as possible taking into account their frequency in the entire
assemblage. As a precautionary measure after each washing procedure the sieves are
brushed, blown with compressed air, submerged in a solution of methylene blue and
dried. The methylene blue marks any foreign object that remained in the sieves
after cleaning so they can be removed or recognized as contamination in
subsequent preparations.
Hard rocks (limestones, sandstones) require the preparation of thin
sections. The foraminifers are then seen as sections, either through an optical microscope or a low-power binocular microscope. In most cases the sections are ground to thicknesses of
approximately three hundredths of a
millimeter (30 µm), in order to preserve sharpness of outline and readability of
the internal structures of the test. Extraction of whole tests using acetic acid rarely gives good results and is seldom
attempted. Less indurated rocks
(marl, chalk) may be washed after crushing, soaking and after heating the
samples treated by hydrogen
peroxide.
An ordinary scanning electron microscope is used routinely for illustrations and
study at high magnifications. Using double-faced adhesive tape the foraminifers are
placed on a metal plate and under vacuum are coated with a thin film of gold
or carbon. The environmental electron microscope does not require a prior
metallization, but currently not all research centers have one. Digital imaging helps greatly in making the photographic plates that are
requisite adjuncts of articles published in micropaleontologic journals. Digital
imagery greatly simplifies the production of the plates of photographs that are
obligatory for the articles published in micropaleontogical journals.
IV. Elements of Systematics
In the classification of living entities by Margulis et alii
(1998), the foraminifers (= Granuloreticulata) are one of the thirty branches
of the
"Protoctista". Today
using molecular characters they are placed
in a larger assemblage which groups several unicellular taxa with no apparent morphologic characters in common (Lecointre & Le Guyader,
2001).
A less ultra-modern taxonomy ("not cladistic" and given in all good
encyclopedias) proposes a hierarchy of systematic relationships in the following
succession. The
Order Foraminiferida is the lowest ranking, hence the most precisely definitive
of this hierarchy: Kingdom Protista, Subkingdom Protozoa, Phylum
Sarcomastigophora, Subphylum Sarcodina, Superclass Rhizopoda, Class
Granuloreticulosa.
Most classifications of the forms included in the Order Foraminiferida - here
assigned the rank of class: the Foraminifera -, as in Loeblich & Tappan
(1987), revised by Sen Gupta
(1999), are artificial, based on the nature of the wall, the arrangement of chambers and the type of aperture.
The Order ALLOGROMIIDA includes all those foraminifera with tests of an organic
material. Tests composed of minerals are classed in
15 orders of which the following are the most important:
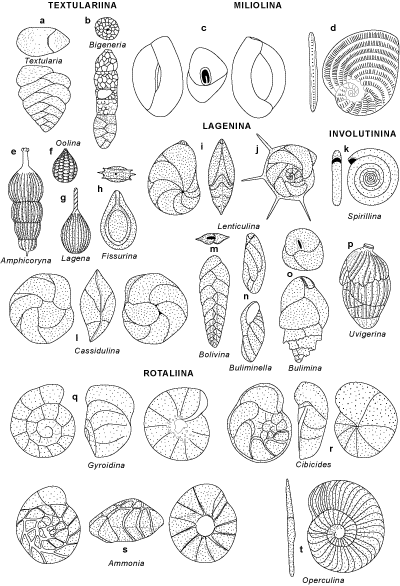
- ASTRORHIZIDA, LITUOLIDA (Orbitolina - Fig. 2
 ), TROCHAMMINIDA
and TEXTULARIIDA (Textularia - Fig. 1a
), TROCHAMMINIDA
and TEXTULARIIDA (Textularia - Fig. 1a  , Bigenerina
- Fig. 1b
, Bigenerina
- Fig. 1b  ): 4
orders that together include all agglutinated foraminifers;
): 4
orders that together include all agglutinated foraminifers;
- INVOLUTINIDA (Spirillina - Fig.
1k
 ) and ROBERTINIDA: 2 reduced suborders that
incorporate all the foraminifers with aragonitic tests;
) and ROBERTINIDA: 2 reduced suborders that
incorporate all the foraminifers with aragonitic tests;
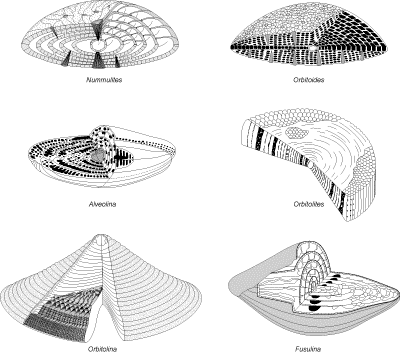
- FUSULINIDA (Fusulina - Fig. 2
 ) houses all foraminifers
(known only from Paleozoic strata) with a
microgranular test;
) houses all foraminifers
(known only from Paleozoic strata) with a
microgranular test;
- MILIOLIDA (Quinqueloculina - Fig.
1c
 , Alveolina
- Fig. 2
, Alveolina
- Fig. 2  ,
Orbitolites - Fig. 2
,
Orbitolites - Fig. 2  , Peneroplis - Fig. 1d
, Peneroplis - Fig. 1d  )
takes in all foraminifers with porcellaneous tests;
)
takes in all foraminifers with porcellaneous tests;
- LAGENIDA (Lenticulina - Fig.
1i-j
 , Amphicoryna
- Fig. 1e
, Amphicoryna
- Fig. 1e  ,
Lagena - Fig. 1g
,
Lagena - Fig. 1g  , Fissurina
- Fig. 1h
, Fissurina
- Fig. 1h  , Oolina
- Fig. 1f
, Oolina
- Fig. 1f  ) comprises those hyaline forms with a special structure
(monolamellar) and radial
aperture;
) comprises those hyaline forms with a special structure
(monolamellar) and radial
aperture;
- BULIMINIDA (B) and ROTALIIDA (R) include most benthic species with hyaline (bilamellar) tests.
These suborders are extremely varied and include a great majority of all benthic
forms.
Some examples of its superfamilies:
- Superfamily Buliminacea (B) (Buliminella - Fig.
1n
 , Bulimina
- Fig. 1o
, Bulimina
- Fig. 1o  ): high trochospiral
tests;
): high trochospiral
tests;
- Superfamily Bolivinacea (B) (Bolivina - Fig.
1m
 , Uvigerina
- Fig. 1p
, Uvigerina
- Fig. 1p  ): biserial
forms;
): biserial
forms;
- Superfamily Cassidulinacea (B) (Cassidulina - Fig.
1l
 ) and Nonionacea
(B): biserial
or planispiral tests;
) and Nonionacea
(B): biserial
or planispiral tests;
- Superfamily Discorbacea (R) (Gyroidina - Fig.
1q
 , Cibicides
- Fig. 1r
, Cibicides
- Fig. 1r  ): low trochospires;
): low trochospires;
- Superfamily Rotaliacea (R) (Ammonia - Fig.
1s
 , Operculina - Fig. 1t
, Operculina - Fig. 1t  , Nummulites
- Fig. 2
, Nummulites
- Fig. 2  ,
Orbitoides - Fig. 2
,
Orbitoides - Fig. 2  ): trochospiral or planispiral with a complex internal
structure.
): trochospiral or planispiral with a complex internal
structure.
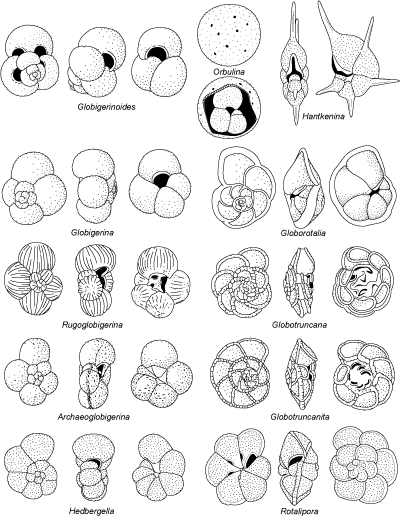
- GLOBIGERINIDA (Fig. 3
 )
includes all planktonic foraminifers (all hyaline bilamellar). Some
examples of its superfamilies:
)
includes all planktonic foraminifers (all hyaline bilamellar). Some
examples of its superfamilies:
- Superfamily Globotruncanacea, found only as fossils, and including bicarenate morphotypes;
- Superfamily Globorotaliacea, trochospiral with few chambers,
its periphery often carinated;
- Superfamily Globigerinacea includes
most forms with globular chambers.
V. The foraminifers and biochronology
1. Biostratigraphic interest of the foraminifers
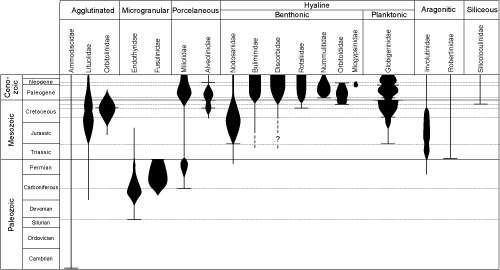
The chronostratigraphic range of the foraminifers extends from the Early
Cambrian to the current epoch (Fig.
5  ). The first forms to appear had organic tests or were simple agglutinated tubes. Until Devonian times species are rare. Microgranular calcitic tests are at a maximum in the Carboniferous and Permian with the development of the Order
Fusulinida that disappears at the end of the
Paleozoic. In Carboniferous strata porcellaneous tests appear with the
Cornuspirids. During Mesozoic times they are followed by many-chambered agglutinated tests
(Lituolids in the Jurassic and Orbitolinids in the Cretaceous) and by the radiation of hyaline tests
(among which are the Nodosariids in the Jurassic). The forms first to appear are all benthic. Planktonic forms do not exist before the
Jurassic. The high sea levels and warm climates of the Cretaceous favored diversification of plankton
but many of the numerous forms were decimated by the major extinctions at the end of the
Mesozoic. An evolutive explosion occurred at the base of the Cenozoic with the appearance of morphotypes similar to those of
existing plankton. The larger benthic foraminifers also diversified in the Paleogene with the
Alveolinids, the Nummulites and the "orbitoidiforms".
). The first forms to appear had organic tests or were simple agglutinated tubes. Until Devonian times species are rare. Microgranular calcitic tests are at a maximum in the Carboniferous and Permian with the development of the Order
Fusulinida that disappears at the end of the
Paleozoic. In Carboniferous strata porcellaneous tests appear with the
Cornuspirids. During Mesozoic times they are followed by many-chambered agglutinated tests
(Lituolids in the Jurassic and Orbitolinids in the Cretaceous) and by the radiation of hyaline tests
(among which are the Nodosariids in the Jurassic). The forms first to appear are all benthic. Planktonic forms do not exist before the
Jurassic. The high sea levels and warm climates of the Cretaceous favored diversification of plankton
but many of the numerous forms were decimated by the major extinctions at the end of the
Mesozoic. An evolutive explosion occurred at the base of the Cenozoic with the appearance of morphotypes similar to those of
existing plankton. The larger benthic foraminifers also diversified in the Paleogene with the
Alveolinids, the Nummulites and the "orbitoidiforms".
The "orbitoidiforms" have disappeared at the beginning of the
Neogene. The diversity of plankton that diminished at the end of the Cretaceous
period was
reversed by brief recrudescence in the warm periods of the Paleogene and Neogene. The distribution of test types and families during time cannot be used to construct a detailed stratigraphic
scale. Precision can be attained only by working at the species level
and also requires on a very detailed knowledge of the phyletic relationships of the species
(microevolution) in order to calibrate as exactly as possible the ranges of the several
taxa.
Biostratigraphy is the study of sedimentary strata based on their fossil content, rather than on lithology or other geologic parameters. Micropaleontologists
divide sedimentary strata into biozones based on the first and last occurrences of selected
species. These levels of appearance and extinction are called
"datums". The taxa so employed are given a variety of names including "marker", "index", "guide", and
"indicator" (McGowran, 2005).
To be of biostratigraphic value a species must have the following
characteristics:
- A short stratigraphic range;
- An extensive geographic dispersion achieved rapidly;
- Be resistant to taphonomic processes;
- Be identifiable without question (easily recognized).
The identification of zones permits the establishment of a relative age, a chronostratigraphy,
for a sedimentary section. The biozones are based on a known succession of
fossil species set up according to the established modes of biologic evolution
during geologic time. During the last fifty years, the
occurrence of fossils and the successive biozones have been integrated with paleomagnetic data and dating
by radioactive isotopes worldwide. The result is a precisely calibrated geologic
time scale. So geochronology is based on absolute rather than relative
ages. Well-dated biozones can be correlated in widely separated sections where
paleomagnetic and other means of dating are lacking. Thus the time scale can be
applied anywhere in the world. These methods have been put in practice for the
whole of the Phanerozoic Eon (some 540 million years), both on the continents and under the
oceans.
Paleontologists identify different kinds of biozones distinguished according
to the use of one or several species and their stratigraphic ranges to determine
the limits of a zone. Using different types of zones insures the establishment
of discrete stratigraphic intervals, each representing a short fraction of geologic time.
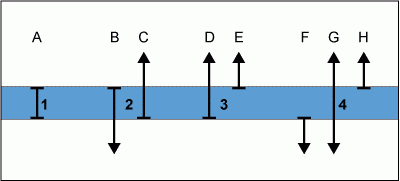
Figure
6  illustrates four different kinds of zones (lines A-H show to the stratigraphic ranges of 8
discrete species). These zones are defined as follows:
illustrates four different kinds of zones (lines A-H show to the stratigraphic ranges of 8
discrete species). These zones are defined as follows:
- Total Range Zone of species A. The entire span of the existence of species A from the appearance of species A until its
disappearance.
- Coincident Ranges Zone of species B and C. That part of the ranges of species B and C from the appearance of species C until the disappearance of species B.
- Partial Range Zone of species D. From the appearance of species D until the appearance of species E.
- Interval Zone of species G. The occurrence of species G between the disappearance of species F and the appearance of species H.
2. Phylogenetic sketch of the plankton
Planktonic foraminifera have been found to be excellent for dating strata
ranging in age from Cretaceous to the Present because of their broad
distribution and their rapid evolution. So they are a first class biostratigraphic
tool (Bolli
et alii, 1985).
These planktonic forms probably derived from benthic foraminifera of which a planktonic phase in the life cycle became completely separate at the beginning of Mesozoic times. This scenario is in
full accord with the fact that the first of both the Jurassic and the
earliest Cretaceous species of plankton are restricted to outer platform
environments (Caron,
1983).
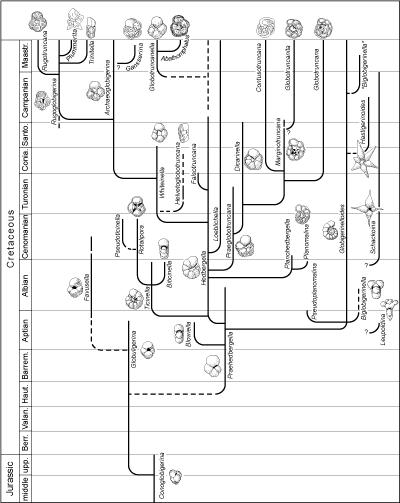
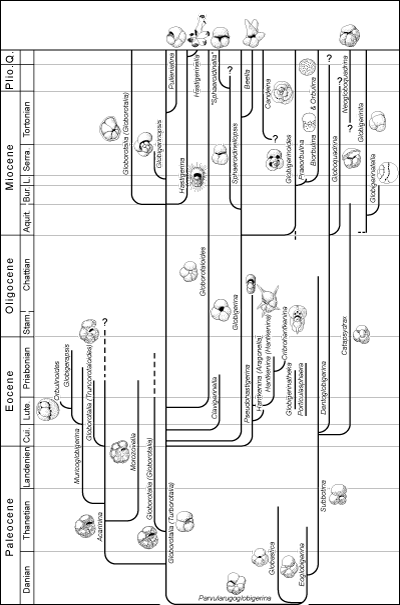
The phylogenetic table of Mesozoic genera (Fig. 7  )
takes into account the rearrangements in taxonomy and classification of the morphotypes of the
Early Cretaceous (Moullade et alii,
2002). The table of Cenozoic genera (Fig. 8
)
takes into account the rearrangements in taxonomy and classification of the morphotypes of the
Early Cretaceous (Moullade et alii,
2002). The table of Cenozoic genera (Fig. 8  ) is less up-to-date because under consideration
is a simplification that a specialist of the matter cannot
accept. Nevertheless, the table presents a reasonably complete picture of evolutionary events
making possible an understanding of "what happened" during the time represented by the interval from the Cretaceous-Paleocene boundary to the
Present. In the two models only the principal trochospiral or planispiral morphotypes are
figured. Not taken into consideration are the morphotypes with serial chambers,
for commonly they are of less interest stratigraphically.
) is less up-to-date because under consideration
is a simplification that a specialist of the matter cannot
accept. Nevertheless, the table presents a reasonably complete picture of evolutionary events
making possible an understanding of "what happened" during the time represented by the interval from the Cretaceous-Paleocene boundary to the
Present. In the two models only the principal trochospiral or planispiral morphotypes are
figured. Not taken into consideration are the morphotypes with serial chambers,
for commonly they are of less interest stratigraphically.
a - The Jurassic "Globigerinomorphs"
Homeomorphs of the globigerines of the Tertiary and Recent appear in the Lias and form a clearly differentiated group in the
Dogger. The tests are small, weakly perforate with a narrow, low-lipped
virguline aperture, not completely
umbilical. The phylogenetic roots of these forms are obscure. As a group they are not very
homogeneous. They have been called "Protoglobigerines" or assigned to the genus
"Globigerina". As Protoglobigerina is a nomen nudum and
because links with similar
Cenozoic morphotypes do not exist, the generic names to designate them are
different: Conoglobigerina for the high-spired forms and
Globuligerina for low-spired ones. In the Early Cretaceous the representatives of this
low-spired group are the source for
Praehedbergella and then Favusella with thickened walls and honeycomb
ornamentation. Both genera are found only in platform environments.
b - The Cretaceous genera
The history of planktonic foraminifera can be used to divide the Cretaceous
into three periods: the time of the Hedbergella and the Rotalipora, of the
Dicarinella and Marginotruncana, and of the Globotruncana and the
Rugoglobigerina.
In the Early Cretaceous planktonic foraminifera are represented principally by relatively unspecialized forms with globigeriniform tests
(the microperforate Praehedbergella, the macroperforate Hedbergella,
etc.). These trochospiral tests with globular chambers are the trunk of the evolutionary
branches. The Hedbergella, in which the umbilical-extraumbilical aperture is edged with a
cuff, comprise a durable very plastic stock of which the diverse tendencies are shown in the different
taxa toward which they
evolve: enrollment becoming planispiral (Globigerinelloides), increase in the number of chambers per whorl and opening of the
umbilicus, then replacement of the lips by "portici" with infralaminal apertures
(Ticinella), extension of the aperture toward the spiral face and the addition of supplementary apertures
there (Loeblichella, American representatives only), displacement of the aperture to a more umbilical position
(Whiteinella).
The addition of a keel is an important phenomenon that marked several
branches at about the same time (note
that the first keel appears previously in Pseudoplanomalina). So successively are distinguished
Planomalina, Rotalipora with true supplementary apertures, Praeglobotruncana
and Helvetoglobotruncana. To Rotalipora is linked Pseudoticinella on which the keel fades out on the last
whorl. The representatives of these last two genera disappear brusquely in the
zone with "large Whiteinella" located at the Cenomanian-Turonian transition.
Shortly before and after this CT event, important mutations appeared in the
several lineages. They produced individual characteristics almost all of which appear again in the Globotruncanas of the Late
Cretaceous. The main steps in this evolution that began with the genus
Praeglobotruncana can be summarized as follows: the appearance of 2 keels (Dicarinella), elongation of the chambers in the direction of enrollment and curvature of the septal sutures of the
umbilical side
(Marginotruncana), migration of the principal aperture toward the umbilicus and the development of umbilical plates,
i.e. "tegilla" (Globotruncana).
Some benthic forms develop an
umbilical plate that separates the umbilical cavity from the chamber lumen by
forming a septal flap, a structure which restricts communication between the chamber and the
umbilical space to one or several small passages called 'loopholes'. In
planktonic forms, there is an umbilical cover called a tegillum that partially
covers and protects the open umbilical cavity.
The same process that led to Globotruncana caused the differentiation of the genus
Archaeoglobigerina. In Rugoglobigerina the chambers are ornamented by radial ridges typically aligned toward their peripheral pole. That stock is affected by variations leading toward the acquisition of a keel
(Rugotruncana), compression (Trinitella), radial elongation (Plummerita). If the affinities of
high-spired
Contusotruncana are detectable, those of the single-keeled Globotruncanita and the origins of
Globotruncanella and Abathomphalus in which the aperture moves to an extraumbilical position are difficult to
elucidate.
From an ecologic standpoint the least specialized (Hedbergelliforms) were able to adapt to very diverse
environments, although all are marine. Their habitat could equally well be the high seas or the continental
platform; they are known in both the intertropical zone and in boreal regions. Keeled and ornamented forms were certainly less
tolerant. The distribution by latitude of the majority was relatively narrow (intertropical and temperate zones). The massive extinction at the end of the Cretaceous affected mainly the highly ornamented and
very strongly keeled forms.
A competent specialist recognizes about a hundred species in the Albian-Maastrichtian succession that make it possible to delimit 25 zones, with an average length of about 1.75 million
years.
c - Cenozoic genera
In following the history of planktonic foraminifers during the Cenozoic, after the end-Cretaceous catastrophe there
was a time of renewal: "dwarf forms" are succeeded by
Acarinina and Morozovella and then Globigerina and
Globigerinoides.
The Tertiary species presumably issued from a resistant line, probably
unspecialized, the only one capable of surviving a brutal change in environment caused by
a combination of catastrophes. A fauna of small Globigerina-like morphotypes with a very simple structure
(Parvularugoglobigerina, Eoglobigerina, Globastica) began to develop at the dawn of the
Paleocene. The rapid development and diversification of species started with the globigerinomorphs
(Subbotina) and unornamented Globorotalia (Turborotalia), followed in the
Middle and Late Paleocene by keeled Globorotalia (Globorotalia sensu stricto) and forms with a spiny surface (Acarinina and Morozovella). This tendency strengthens in the
Early Eocene (appearance of Globigerina, Truncorotaloides, Catapsydrax, etc.) and accelerates in the
Middle and Late Eocene where several new genera appear
(Hantkenina, Cribrohantkenina, Globigerinatheca, Globorotaloides,
Orbulinoides, etc.).
The development of this characteristic fauna was brutally interrupted at the end of the
Eocene. Those extinguished then were mainly very evolved and had had a short
range, while several species of globigerines continued into the Oligocene. Here too a sudden ecologic change, probably a rapid lowering of
temperature, caused the disappearance of the highly specialized species.
Beginning in the Early Oligocene, for the third time (as it had in the Early Cretaceous and
Early Paleocene) a fauna developed from simple Globigerina-like forms, unkeeled Globorotalia and
later, in the Miocene, from keeled Globorotalia. In the Early Miocene Globigerinoides,
Globoquadrina and Globigerinita appeared. During the Neogene numerous specialized forms
manifested themselves (for example
Globigerinatella, Sphaeroidinellopsis, Hastigerina, Candeina,
Orbulina, etc.). This tendency to diversification that began in the
Early Oligocene continues today.
In Paleocene times, the distribution of taxa is uniform worldwide up to latitudes in the neighborhood of 50°.
Changes in climate had an effect on this broad geographic distribution, first at the end of the
Paleogene, and in a much more pronounced way during the Neogene. As a
consequence, the associations of foraminifers became segregated according to climatic
zone: tropical-subtropical, temperate and cold. Although there were overlaps in these zones, correlation on an interregional scale becomes
difficult. This is particularly true for the Miocene.
As in the Cretaceous, the distribution of planktonic foraminifera in the Cenozoic permits its division into some forty zones with an average length of 1.75 million
years.
VI.
Foraminifers and (paleo-) environments
1. Foraminifers and oceanic deposits
The oceans cover 70% of the planet's surface. They are a great trough in which all of the materials
washed seaward from the continents collects. But the trough also produces
autochthonous sediments, derived principally from the life that inhabits it.
a - Marine environments
The margino-littoral or peritidal domain is a maritime border zone in which the salinity
of water varies (influx of fresh water and/or isolation from the sea). The plateau or continental platform
(0 to -200 meters) is the site of the euphotic zone (or zone of light-seeking
algae), its lower limit set by the greatest depth to which light penetrates. It is
equivalent to the littoral domain (s.l.) divided into infra- and
circa- littoral zones (in which live the algae requiring little light). The infralittoral domain is one of agitated waters; it is strongly influenced by seasonal variations
in temperature (seasonal thermoclines). The bathyal stage includes the continental slope (or talus) and the glacis to a depth of about
3,000 meters. Areas in which the depth is more than 3,000 m (the great abyssal
plains) are assigned the abyssal stage.
b - Terrigenous oceanic deposits
Sedimentation on the continental margin is mainly terrigenous, that is,
its deposits are produced by erosion of the continents. More or less altered rock debris is transported, accumulates, is cemented to varying degrees, and
forms detrital sedimentary rocks. Their make-up
is disparate. It depends on the original nature of the rock
eroded, the climate of the source area, the hydro- or aero- dynamic conditions of transport and
deposit, and on post depositional transformations (diagenesis).
The average grain size is a function
of its ultimate location: without taking into account turbidite, tsunami and contour current deposits,
the coarsest grains are on the
beach (pebbles, coarse sand) ranging down to mud- and clay- size when the depth of water
is greater than that of the wave base during storms
(around 100 meters).
c - Biogenic oceanic deposits
Plankton are one of the essential elements of the oceans. They consist of the assemblage of micro-organisms that live at the surface of the
oceans, in a layer that ranges up to several tens of meters in thickness and in some places
attains more than 100
meters; it is a true organic soup. A considerable percentage of planktonic organisms have a mineral skeleton either of calcium carbonate
(CaCO3 calcite or aragonite) e.g., the foraminifers and certain micro-algae of the
nannoplankton, or of silica (SiO2) e.g., the diatoms and radiolaria.
When an individual dies its skeleton becomes a particle of
sediment. Consequently the surficial levels (i.e. the euphotic zone) of the oceans
produce a continual rain of
particles. This rain, made up of unoxidized organic matter, limestone and silica forms a
stratum of very fine-grade sediment on the ocean
floor. But there is a natural limit called the CCD (calcite compensation
depth) below which calcite is dissolved. Seaward of the continental margins most
sediments are produced
biologically by the ocean itself. On the abyssal plains and ridges these sediments form a layer composed of a mixture of organic
matter, silica and possibly carbonates, with variable amounts of clays and atmospheric
dust.
The major constituents of oozes (or muds) are pelagic carbonates that in true
open marine environments are the tests of foraminifers and the plates of coccolithophorids
with a calcite test and pteropod shells with an aragonite test that is preserve only in shallow
water. In shallow milieus close to the coast a complete cortege of macro-organisms
(mollusks, gastropods, echinoderms, etc.), encrusting algae and ooids
(in the tropical realm) accompany the carbonates of planktonic origin.
Benthic foraminifers prefer the relatively shallow waters of continental platforms where they proliferate under certain conditions
(adequate food supply or lots of light, etc.)
There is a close relationship between the morphology of the test and the mode of life. The forms that live in areas
of waves and currents are heavily built with strongly calcified
shells.
Some foraminifers live in symbiosis with algae. So there is a relationship between the kind of test and
the intensity of light: in the porcellanids the light is filtered
because it is too strong (these foraminifers are adapted to live in very shallow
water, predominantly in sunny tropical areas); in the hyalinids the light rays may be directed,
an adaptation to a lower intensity.
In general, spherical or lenticular forms (biconvex, symmetrical) remain at the surface of the substratum; fusiform foraminifers are
diggers; the asymmetric trochospiral forms with a flat face are attached; flattening of the test generally
increases with depth. There is also a relationship between depth and
ornamentation: In shallow waters the test often has numerous nodules and spines to protect against abrasion.
Planktonic foraminifera require stable physico-chemical conditions and in particular do not tolerate harmful effects caused by
an influx of continental waters (that produces turbidity, lowering of salinity, etc.). They are well adapted to
floating: thin tests, fatty protoplasmic inclusions, gas-filled capsules,
etc.
Living forms with spiny tests (Globigerina, etc.) do well in
near-surface waters; those with smooth tests
(Globorotalia, etc.) begin their life cycle near the surface before plunging afterward to a depth of several tens or hundreds of
meters.
As coined by L. Hottinger
(personal communication, 26-06-2009), the benthos-plankton ratio
in sediments is linked with the life cycle of the planktonic foraminifers and in
particular with the depth at which reproduction takes place. The importance of
the procedures involving the loss of spines during the descent in the water
column must not be overlooked. So a large number of planktonic tests appear in a
depth gradient only where "mother" tests, empty after reproduction,
are deposited after the phase of reproduction ends. They do not indicate where
the species was living but more precisely where it reproduced.
Certain species are ubiquitous. The distribution of others is restricted to
certain latitudes.
2. Determination of environmental
conditions using foraminifers
The conditions necessary to the existence of most taxa are well-defined, so
they can be used as indicators of environment
(mainly bathymetry), but
taxonomic knowledge about the foraminifers suitable for this purpose cannot be acquired in a short time.
But the following general characteristics are important (Sartorio & Venturini,
1988):
- Only a restricted number of species of benthic foraminifera exist in lagoonal
environments. Genera typical of this milieu:
Alveolinella, Ammobaculites, Peneroplis, Trochammina;
-
In continental seas benthics dominate with species in numbers that are
relatively important. Some genera typical of proximity to shore are:
Ammonia, Elphidium, Quinqueloculina; on the inner platform
occur:
Discorbinella, Eponides, Lenticulina, Textularia; in the middle of the
platform appear: Amphicorina, Bigenerina, Lenticulina; and on the outer
platform;
Bolivina, Bulimina, Discorbis, Nodosaria, Uvigerina,
Globigerina;
-
In the bathyal domain the assemblages are mainly planktonics (Globigerina, Globigerinoides, Globorotalia, etc.); typical benthonic
taxa:
Epistominella, Nodosariidae, Pyrgo;
-
In the abyssal domain simple agglutinated forms predominate.
a - Large foraminifers and their paleoenvironments
The current distribution of large foraminifera along a continent to deep-ocean profile
offers models of ecologic distribution that can be
transferred, albeit with great precaution, to certain past domains at specific geologic
periods.
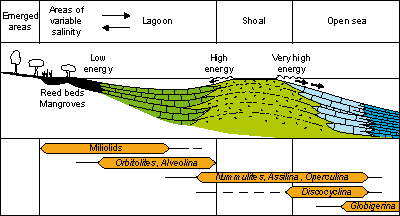
Among them, Arni's model (1965,
see Fig.
9  )
is the most classic for the Tethyan Eocene. In addition to algae he uses the large porcellaneous and
large benthic forms to which he adds miliolids and plankton. Using only
"relatively simple" supra-specific characteristics this model permits the positioning of almost any assemblage in the succession of paleoenvironments from the coast to the open
sea. It may be applied in a broad sense and with prudence to all Cenozoic carbonate
platforms. It is a practical tool for rapid evaluation of the depth, salinity, oxygen content and energy of lagoonal
and oceanic waters .
)
is the most classic for the Tethyan Eocene. In addition to algae he uses the large porcellaneous and
large benthic forms to which he adds miliolids and plankton. Using only
"relatively simple" supra-specific characteristics this model permits the positioning of almost any assemblage in the succession of paleoenvironments from the coast to the open
sea. It may be applied in a broad sense and with prudence to all Cenozoic carbonate
platforms. It is a practical tool for rapid evaluation of the depth, salinity, oxygen content and energy of lagoonal
and oceanic waters .
b - Small foraminifers and paleoenvironments
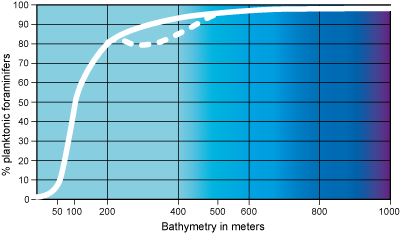
Index of oceanity
The index of oceanity of a foraminiferal population (Gibson, 1989)
is the quantitative relationship between the number of plankton and the total number of individuals (benthic +
plankton) in the sediment
i = P / P + B
P is the number of planktonic foraminifers in a defined volume of sediment; B is the number of benthic foraminifers in the same volume. The total of P+B should be between 100 and 300.
Optimum productivity for planktonic foraminifers is in marine waters far from a
coast. Benthic foraminifers proliferate on continental platforms. So the index of oceanity permits the evaluation of bathymetry and consequently the
several environments encountered in a profile embracing the tracts from the continental platform to the abyssal
domain.
Studies carried out in different regions of the Globe show that a proportion of 50% planktonic foraminifera
occurs at a depth between 100 and 200 meters. It equates with the outer zone of the continental
platform (circalittoral stage). The proportion increases very rapidly at the
outer limit of the platform, and at depths greater than 200 m the index of oceanity is more than 80% (Figs. 10  -
11
-
11  ).
).
This first index permits the recognition of a frankly marine environment, and of environments with a continental influence
(estuaries, lagoons, etc.).
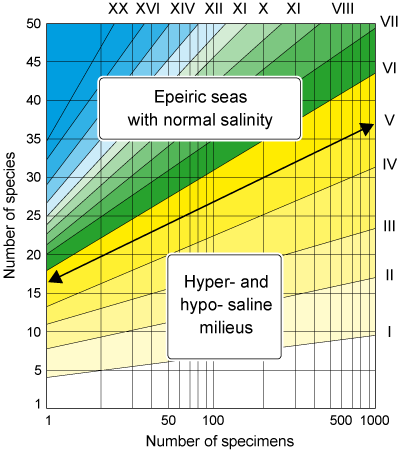
Index of diversity and strategies for living
This second index permits recognition of the several environments under the influence of the
continent (Murray,
1991); consequently only benthic foraminifers are
employed in its calculation.
The best way to express specific diversity in a foraminiferal population is to determine the relationship between the
number of individuals and the number of species to which they
belong. In it simplest form specific diversity is the number of species.
Calculation of the index of diversity may be carried out mathematically but in practice the index values are read on an abacus or the number of individuals is carried as an abscissa and the number of species as an ordinate
(Fig. 12  , indices in Roman
numerals). Continental seas of normal salinity are thus distinguished quite clearly from
hypo- and hyper- saline lagoons and swamps, the distinction being the line of diversity of index V.
, indices in Roman
numerals). Continental seas of normal salinity are thus distinguished quite clearly from
hypo- and hyper- saline lagoons and swamps, the distinction being the line of diversity of index V.
The habitat of a species controls its demographic strategy. Stable permanent habitats have predictable characteristics in contrast to unstable habitats, like seas or
lagoons. The species with strategy r of unstable habitats are called opportunists and the species of strategy
K of stable habitats are species in equilibrium. Opportunistic species have short
lives; they are small forms of limited growth, with numerous progeny and little diversity. Species in equilibrium grow over a longer time, have a limited number of
offspring, and vary greatly.
Triangular diagram of physico-chemical conditions
In the infra- and circa- littoral domains the relative percentages of hyaline, porcellaneous and agglutinated tests permit an evaluation of water
temperature (Murray,
1991). Thus a simple analysis of the composition of a biocoenosis using the three types of test provides data on ambient
physico-chemical conditions
(Fig. 13  : a point located at the tip of each angle of the triangle indicates that the population includes 100% of the type
involved, on the bases of the triangles 0% of the type indicated in the opposite
angle). These diagrams show clearly that the biocoenoses in which hyalines and porcellanids predominate indicate warm
seas, while hyalines and agglutinates predominate in cold seas.
: a point located at the tip of each angle of the triangle indicates that the population includes 100% of the type
involved, on the bases of the triangles 0% of the type indicated in the opposite
angle). These diagrams show clearly that the biocoenoses in which hyalines and porcellanids predominate indicate warm
seas, while hyalines and agglutinates predominate in cold seas.
c - Planktonic foraminifers, paleogeographies and paleoclimates
The biodiversity of actual living planktonic foraminifers permits the
delimitation of five latitudinal biogeographic provinces in each hemisphere.
Each of these provinces delimits a
corresponding thermal interval, characterized by a specific index association (Bé & Tolderlund,
1971; supplements in Hembelen et alii,
1989). Figure
14  explains their relationships:
explains their relationships:
- tropical (1: 0 to 25°N and 0 to 10°S; temperatures from 24 to
30°C);
- subtropical (2: 25 to 40°N and 10 to 30°S; temperatures from
18 to 24°C);
- transitional (3: 40 to 45°N and 30 to 40°S; temperatures from
10 to 18°C);
- subpolar (4: 45 to 60°N and 40 to 50°S; temperatures from 5 to
10°C);
- polar (5: 60 to 90°N and 50 to 90°S; temperatures from 0 to 5°C).
This current demarcation into five well-defined bands becomes progressively
less clear-cut with regression into the several epochs of the Cenozoic. In the Mesozoic the thermal distinctions are less marked but
for the Cretaceous period the
planktonic foraminifers are still discriminatory. The paleoprovincial reconstructions concerning this
period published at the end of the last century (compiled in Hart,
2000) do not take into account the precise specific
makeup of these associations. Using general morphologic distinctions they
consider only the relative percentages of forms with globular chambers
and those with keels.
Relationships of planktonic Foraminifera during the latest Albian can be used as an example. It was a key period in
the evolution of the lineage during which robust keels appeared on
the tests. Using this development, in each hemisphere three biogeoprovinces were
distinguished (Fig.
15  ):
):
- undivided Tethyan province (1 = tropical: 0 to 40°N);
- transitional province (2: 40 to 50°N and 0 to 45°S);
- boreal or austral province (3: 50 to 90°N and 45 to 90°S).
During this same period species ranges revised by data from
deep sea wells "DSDP" (Deep Sea Drilling Project) and "ODP"
(Ocean Drilling Program) were taken into account.
They demonstrated a subdivision of the Tethyan province into three latitudinal
belts,
the limits of which were drawn at about 25 and 30°N
(Bellier & Vrielynck,
2007). In the three subdivisions of this
tropical province, the associations are made up of keeled and unkeeled
morphotypes, neither predominant. In the Atlantic waters of today such a
cohabitation is characteristic of tropical, subtropical and transitional domains, that is a latitudinal tract between
45°N and 45°S. Paleobotanical
data confirm that during the Albian (Chumakov, 1995, in Skelton et alii,
2003)
there were three belts from the equator to the pole: an equatorial arid belt, a
warm belt in the middle latitudes and a temperate belt in the high latitudes. If
the distribution of Cretaceous foraminifers is related to differences in
temperature, as is now the case, the plankton of the Late Albian
recorded oceanic paleoprovinces narrower than those indicated by terrestrial
plants. If so, microplankton are among the most sensitive indicators
of paleoclimates.
The transition from Albian to Cenomanian may have been marked by important
fluctuations in temperature, according to the measurements of δ18O
in belemnites and mollusks (Frakes, 1999):
the Albian is one of the first warm episodes. It forecasts the hothouse effect during the Late Cretaceous,
that followed a very short cold
episode during the transition from latest Albian to earliest Cenomanian.
Planktonic foraminifers are surely a good choice for isotopic analyses, but the
data in the interval under consideration are rare and of no value because they are
contradictory. If only paleoenvironmental criteria are
taken into account (Hart,
2000), the habitats indicated by the measurements agree
only exceptionally with what is known about the paleodepths
attributed to the several taxa. Variations in the distribution of all
northern Atlantic species during the Albian-Cenomanian transition make apparent
an increasing biodiversity. This increase is inferred to imply a warming
episode. But the species (only 18) that survived the transition there is no appreciable displacement
equatorward of the range of the taxa to the South (Bellier
& Vrielynck,
2007). So the planktonic foraminifers did not
register the cooling indicated by isotopic measurements made on
invertebrates.
It is interesting to observe that the dinoflagellate biogeoprovinces of
the Late Albian have a line of separation between 25 and 30°N
that traces the limit between an equatorial tropical domain occupied by
endemic and cosmopolitan taxa and a subtropical domain in which only
cosmopolitan taxa are present (Masure et alii,
2003). This transitional front, which is seen in two
discrete micropaleontologic groups, botanical and zoological,
thus appears to be indicative of a paleoclimatic reality.
Conclusion
The foraminifers, an important fraction of oceanic productivity, are indicators of both environment and age and are often available in large
quantities. These qualities are particularly helpful in several fields: fundamental research
work, geologic mapping, petroleum exploration and oceanic drilling.
In marine scientific campaigns, the utility of foraminifers is
exemplified: it is the foraminiferal specialists associated with nannofossil
experts who
determine the relative ages of the sedimentary sequences penetrated during well drilling
operations. After every 9.5 m in the case of the JOIDES Resolution
drilling platform, for instance, the core barrel is brought up from the sea floor and a sample from the "core catcher", the
"cork" that keeps the core from sliding out of the tube, is cut up and shared between the two groups of
specialists. Mounts of "smears" between glass slides are prepared for the study of the calcareous nannofossils, and a larger portion is washed to extract the
foraminifers; both are routine operations. The determination of age is obtained in less than an
hour, sometimes in less than 15 minutes. The rapid establishment of a chronostratigraphy is fundamental to all
shipboard analyses, particularly during those expeditions intended to locate critical events in earth history like the
Cretaceous-Paleogene boundary or the thermal maximum of the
Paleocene-Eocene. Thus, drilling strategies and programs can be adjusted, if necessary, to lessen the risk that the
level of interest be placed erroneously or lost between two cored
intervals.
The recognition of biostratigraphic zone helps a great deal in the identification of
magnetic inversions that then become correlatable with the chrons of the
scale of geomagnetic polarity. Biostratigraphy also has a key role in developing
a model for the age of each of the sedimentary sequences drilled; diagrams of age versus depth in the wells permit
calculation of the rates of sedimentation. The control of relative age and an approach to absolute age are necessities in order to fix in time all types of data and to understand how the strata traversed are situated in the general
reference scale of time.
Acknowledgments
We (J.-P.B. and R.M.) have only the warmest memories of our close cooperation throughout these many long years with our former colleagues and friends Françoise Dépêche, Claude Guernet
and Gérard Bignot (†), of our relationships, long-lasting or ephemeral, with the
teachers-searchers, searchers and thesis-students of the Paris Micropaleontology Laboratory, who lived through the peregrinations of the said laboratory from the prefabs of the Rue St. Jacques to the towers 15-25 and then
46-56 of the Jussieu University campus We also express our appreciation to
L. Hottinger and to M. Moullade
for suggestions that helped to improve a preliminary version of our manuscript.
Planned initially as a chapter of a Manual of Micropaleontology,
written by the same authors supplemented by other specialists, for legal and
subordinate technical reasons it is being published before the book appears.
Legal matters concern certain illustrations that we had hoped to use. Not
holding rights to them we requested authorization for reproduction from their
authors and editors. We thank M. Hart, along with the
B.R.G.M. (Orleans),
Elsevier (Oxford), Geological Society Publishing House (London), Koninklijke
Brill (Leiden), Zitteliana (Munich) and their respective representatives: F.
Trifigny, C. Truter, A. Hills, G. van Rietschoten, M. Krings, for having granted
these permissions or for having assisted us in our efforts. However, it soon
appeared that it was not feasible to track down rights to all of the figures.
For this reason we opted to withdraw all of these original figures and to
replace them by "new" illustrations, certainly of the same nature as
those previously selected but with significant modifications. For them, our
thanks go to Alexandre Lethiers
and Claude Abrial of the Design Studio of the Pierre and Marie
Curie
University, talented draftsmen
respectively responsible for figures 1-3 & 5-13 and figures 14-15. Last but not
least, Nestor Sander provided invaluable help with
contributions to the English version of both the text and figures.
Bibliographic references
Arni P. (1965).- L'évolution des Nummulitinae en tant que facteur de modification des dépôts littoraux. In: Colloque International de Micropaléontologie (Dakar, 6-11 mai 1963).- Mémoires du Bureau de Recherches et Minières, Orléans, N° 32, p. 7-20.
Bé A.W.H. & Tolderlund D.S. (1971).- Distribution and ecology of living planktonic foraminifera in surface waters of the Atlantic and Indian Oceans. In: Funnell B.M. & Riedel W.R. (eds.), The micropalaeontology of oceans.- Cambridge University Press, p. 105-149.
Bellier J.-P., Dépêche F. & Mathieu R. (1995).- Introduction à la Micropaléontologie.- Documents pédagogiques du Laboratoire de Micropaléontologie, Université Pierre et Marie Curie, Paris, N° 1, 50 p.
Bellier J.-P. & Vrielynck B. (2007).- Distribution des foraminifères planctoniques au passage Albien-Cénomanien dans l'Atlantique Nord : Indices de l'existence d'une zonation latitudinale dans la province téthysienne.- Revue de Paléobiologie, Genève, vol. 26, n° 1, p. 55-62.
Bignot G., Dépêche F. & Mathieu R. (1975).- Initiation pratique à la Micropaléontologie.- Travaux du Laboratoire de Micropaléontologie, Université Pierre et Marie Curie, Paris, N° 4, 217 p.
Bignot G. (2001).- Introduction à la micropaléontologie.- Gordon and Breach Science Publishers, Paris, 258 p.
Blow W.H. (1979).- The Cainozoic Globigerinida: A study of the morphology, taxonomy, evolutionary relationships and the stratigraphical distribution of some Globigerinida (mainly Globigerinacea).- 3 vols., E.J. Brill, Leiden, 1413 p.
Bolli H.M.,
Saunders J.B. & Perch-Nielsen
K. (1985).- Plankton stratigraphy.-
Cambridge University Press, 1032 p.
Caron M. (1983).- Taxonomie et phylogénie de la famille des Globotruncanidae. In: 2. Symposium Kreide. München 1982.- Zitteliana, München, (Reihe B: Abhandlungen der Bayerischen Staatssammlung für Paläontologie und Geologie), 10, p. 677-681.
Chumakov N.M. (1995).- Climatic zones in the middle of the Cretaceous Period.- Stratigraphy and Geological Correlation, Moscow, vol. 3, p. 3-14.
Frakes L.A. (1999).- Estimating the global thermal state from Cretaceous sea surface and continental temperature data. In: Barrera E. & Johnson C.C. (eds.), Evolution of the Cretaceous ocean-climate system.- Geological Society of America, Special Paper, Boulder, vol. 332, p. 49-57.
Gibson T.G. (1989).- Planktonic
benthonic foraminiferal ratios: modern patterns and Tertiary applicability.- Marine Micropaleontology, Amsterdam, vol.
15, n° 1-2, p. 29-52.
Hart M.B. (2000).- Climatic modelling in the Cretaceous using the distribution of planktonic Foraminiferida. In: Hart M.B. (ed.), Climates: Past and Present.- Geological Society, Special
Publication, London, n° 181, p. 33-41.
Hart M., Hudson W. & Smart C.W. (2007).- Palaeobiogeography of early planktonic foraminifera.- 1er Symposium international de Paléobiogéographie (Paris, 10-13 juillet 2007), Résumés, p. 47.
Hembelen C., Spindler M. & Anderson O.R. (1989).- Modern planktonic foraminifera.- Springer-Verlag, New-York, xiv + 363 p.
Hottinger L. (2006).-
Illustrated glossary of terms used in foraminiferal research.- Carnets
de Géologie / Notebooks on Geology, Brest, Memoir 2006/02 (CG2006_M02),
126 p.
Langer M.R. (1999).- Origin of foraminifera: Conflicting molecular and paleontological data?.- Marine Micropaleontology, vol. 38, p. 1-5.
Lecointre G. & Le Guyader H. (2001).- Classification phylogénétique du Vivant.- Ed. Belin, 544 p. + annexes.
Lister J.J. (1895).- VIII. Contributions to the life-history of the foraminifera.- Philosophical Transactions of the Royal Society of London B, vol. 186, p. 401-453, pls. 6-9.
Loeblich A. & Tappan H. (1987).- Foraminiferal genera and their classification.- Van Nostrand Reinhold, New York, vol. 1, 970 p.; vol. 2, 212 p. + 847 pls.
Margulis L., Gould S.J., Schwartz K.V. & Margulis A.R. (1998).- Five kingdoms: An illustrate guide to the phyla of life on Earth.- Freeman & Co, New York, 448 p.
Masure E., Vrielynck B. & Fiet N. (2003).- Les Dinoflagellés et le gradient de température des eaux océaniques de surface à l'Albien supérieur. In: Bassins crétacés de France et d'Europe occidentale.- Séance spécialisée de la Société Géologique de France, 6-7 novembre 2003, résumé.
Mathieu R. (1986).-
Sédiments et foraminifères actuels de la marge continentale atlantique du Maroc.-
Thèse de Doctorat d'État ès Sciences naturelle, Université Pierre et Marie Curie, Paris 6;
Mémoires des Sciences de la Terre, Paris, n° 86-14, 419 p.
Mathieu R. (1988).- Foraminifères actuels et résurgences côtières sur la marge continentale atlantique du Maroc. In: Benthos'86.- Revue de Paléobiologie, Genève, vol. spécial, n° 2, Partie II, p. 845-850.
McGowran B. (2005).-
Biostratigraphy. Microfossils and geological time.- Cambridge University Press, 480 p.
Moullade M., Bellier J.-P. & Tronchetti G. (2002).- Hierarchy of criteria, evolutionary processes and taxonomic simplification in the classification of Lower Cretaceous planktonic Foraminifera.- Cretaceous Research, London, vol. 23, n° 1, p. 111-148.
Murray J.W. (1991).- Ecology and palaeoecology of benthic foraminifera.- Longman Scientific & Technical, 397 p.
Pawlowski J., Holzmann M., Berney C., Fahrni J., Gooday A.J., Cedhagen T., Habura A. & Bowser S.S. (2003).- The evolution of early Foraminifera.- Proceedings of the National Academy of Sciences of the United States of America, Washington, vol. 100, n° 20, p. 11494-11498.
Sartorio D. & Venturini S. (1988).-
Southern Tethys biofacies.- Agip, San Donato Milanese, 235 p.
Sen Gupta
B.K. (1999).-
Systematics of modern Foraminifera. In: Sen Gupta
B.K.
(ed.), Modern Foraminifera.- Kluwer Academic Publishers, Dordrecht, p. 7-36.
Skelton W., Spicer
R.A., Kelley S.P. & Gimour L. (2003).- The Cretaceous world.- The Open University, Cambridge University Press, 360 p.
Figures
Click on the image to enlarge it.
Figure
1: Small benthic foraminifers (their largest dimension on the order of a half-centimeter).
Click on the image to enlarge it.
Figure
2: Some large benthic foraminifers (their largest dimension on the
order of a centimeter).
Click on the image to enlarge it.
Figure
3: Some planktonic foraminifers (their largest dimension on the order
of a half-millimeter).
Click on the image to enlarge it.
Figure
4: The reproductive cycle of benthic foraminifers (illustrated by Elphidium
crispum (Linné), ex "Polystomella crispa",
after J.J. Lister, modified).
Click on the image to enlarge it.
Figure
5: Stratigraphic ranges of some families of foraminifers (after Bignot,
2001, modified).
Click on the image to enlarge it.
Figure
6: Principal types of zones.
Click on the image to enlarge it.
Figure
7: Phylogeny of Mesozoic planktonic genera (after Caron,
1983, modified).
Click on the image to enlarge it.
Figure
8: Phylogeny of Cenozoic planktonic genera (after Blow,
1979, modified).
Click on the image to enlarge it.
Figure
9: Ecologic zonation by foraminifers of Mesogean platforms during
the Eocene (about 40 to 50 Ma) (after
Arni, 1965, simplified).
Click on the image to enlarge it.
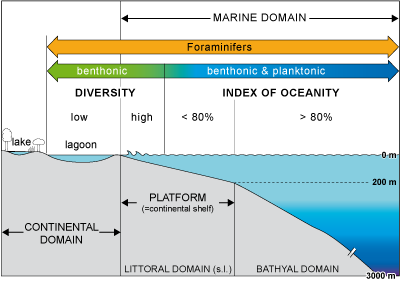
Figure
10: Index of oceanity, diversity of Foraminifera and (paleo-)
environments.
Click on the image to enlarge it.
Figure
11: Index of oceanity (after Gibson, 1989,
simplified). On continental margins subject to upwellings the percentage curve
of planktonic foraminfers is inflected (dotted curve) at the upper edge of the
continental slope (between 200 and 500 meters). It is in connection with an
important rise in the number of benthic species (after Mathieu,
1988).
Click on the image to enlarge it.
Figure
12: Index of diversity (after Murray,
1991): hyposaline ‹ 33‰, normal = 33-37‰,
hypersaline › 37‰.
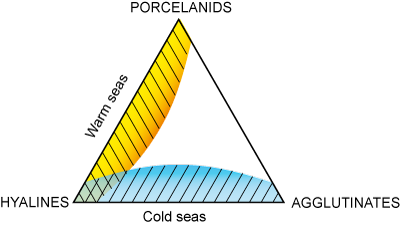
Click on the image to enlarge it.
Figure
13: Placement on a triangular diagram of the biocenoses of the infra-
and circa- littoral domains (after Murray,
1991).
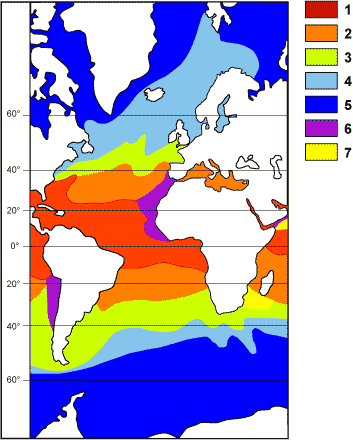
Click on the image to enlarge it.
Figure
14: Biogeograhic provinces as delimited by the biodiversity of living
planktonic foraminifers (after
Bé
& Tolderlund, 1971; Hemleben et alii,
1989, modified). 1) tropical province; 2)
subtropical province; 3) transitional province; 4) subpolar province; 5) polar
province; 6) zones of upwelling; 7) tropical/subtropical province.
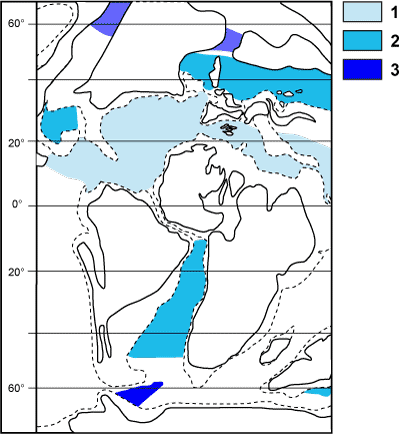
Click on the image to enlarge it.
Figure
15: Paleobiogeographic provinces during the latest Albian using
Cretaceous planktonic foraminifers (after Hart,
2000, modified excerpt): 1) tropical (Tethyan); 2)
transition; 3) boreal (N) and austral (S).
Plates

Click on the image to enlarge it.
Plate 1:
SEM views of representative morphotypes of the genera Spiroplectammina
(a), Bigenerina (b-c), Quinqueloculina (d and g), Parasorites
(e:oblique view; f: lateral view), Borelis (h), Peneroplis (i:
lateral view; j: oblique view), Spirolina (k) - Atlantic margin off
Morocco. Graphical scales = 100 µm.
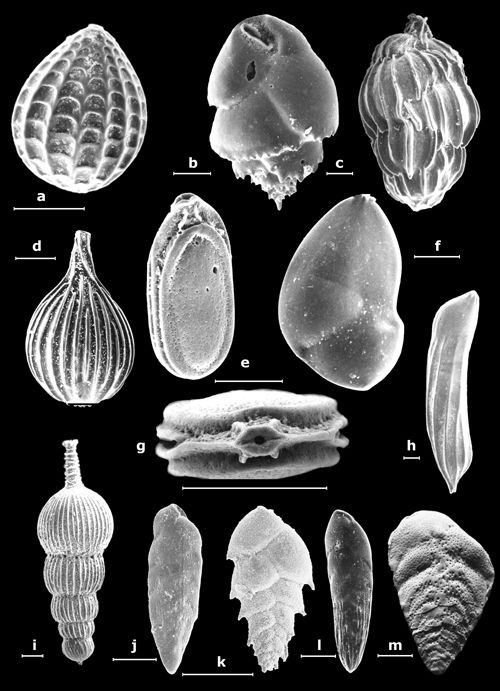
Click on the image to enlarge it.
Plate
2: SEM views of representative morphotypes of the genera Oolina
(a), Bulimina (b: in
Mathieu, 1986, Pl. 8, fig. 10), Uvigerina (c:
lateral view in Mathieu, 1986,
Pl. 9, fig. 10), Lagena (d), Fissurina (e: oblique view; g:
apical view), Lenticulina
(f: in Mathieu, 1986, Pl. 6, fig. 10), Dentalina
(h), Amphicoryna (i), Bolivina (j-m) - Atlantic margin off
Morocco. Graphical scales = 100 µm.
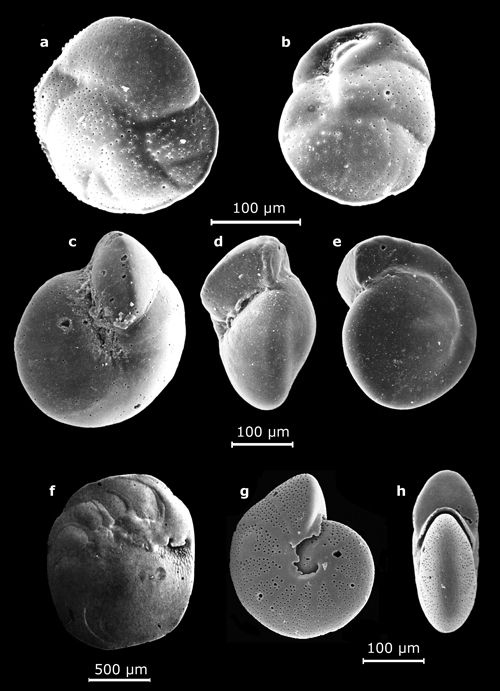
Click on the image to enlarge it.
Plate
3: SEM views of representative morphotypes of the genera Cassidulina
(a: spiral face in Mathieu, 1986,
Pl. 10, fig. 7; b: umbilical face), Gyroidina (c: umbilical face; d:
profile in Mathieu, 1986,
Pl. 17, fig. 5; e: spiral face in Mathieu, 1986,
Pl. 17, fig. 4), Amphistegina (f: umbilical face), Melonis (g:
lateral view in Mathieu, 1986,
Pl. 14, fig. 10; h: profile in Mathieu, 1986,
Pl. 14, fig. 12) - Atlantic margin off Morocco. Graphical scales = 100 µm,
except in f = 500 µm.

Click on the image to enlarge it.
Plate
4: SEM views of representative morphotypes of the genera Heterolepa
(a: umbilical face; b: profile in Mathieu, 1986, Pl.
15, fig. 3; c: spiral face), Ammonia (d: umbilical face; e: spiral
face), Cribroelphidium (f: oblique view), Elphidium (g: lateral
view in Mathieu, 1986, Pl. 16, fig.
10; h: oblique view in
Mathieu, 1986, Pl. 16, fig.
11; i: detail of h) - Atlantic margin off Morocco. Graphical scales = 100 µm.
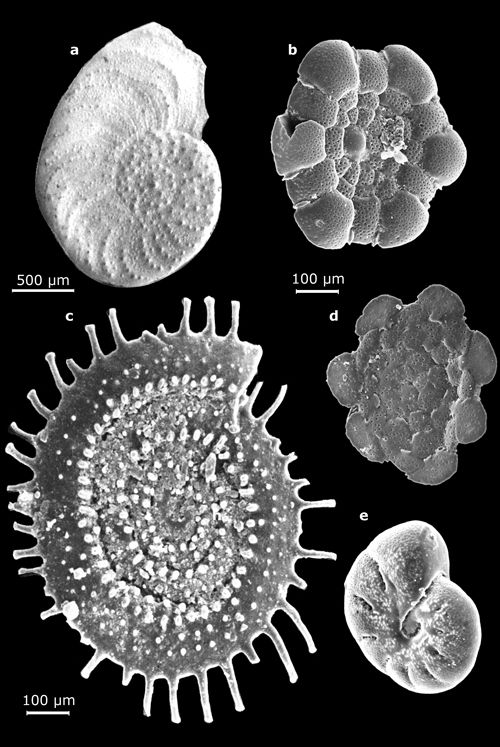
Click on the image to enlarge it.
Plate
5: SEM views of representative morphotypes of the genera Operculina
(a: lateral view), Planorbulina
(b: umbilical face in Mathieu,
1986, Pl. 14, fig. 2; d: spiral face in Mathieu,
1986, Pl. 14, fig. 1), Spirillina
(c: lateral view), Astrononion
(e: oblique view in Mathieu,
1986, Pl. 14, fig. 7) - Atlantic margin off
Morocco. Graphical scales = 100 µm, except in a = 500 µm.
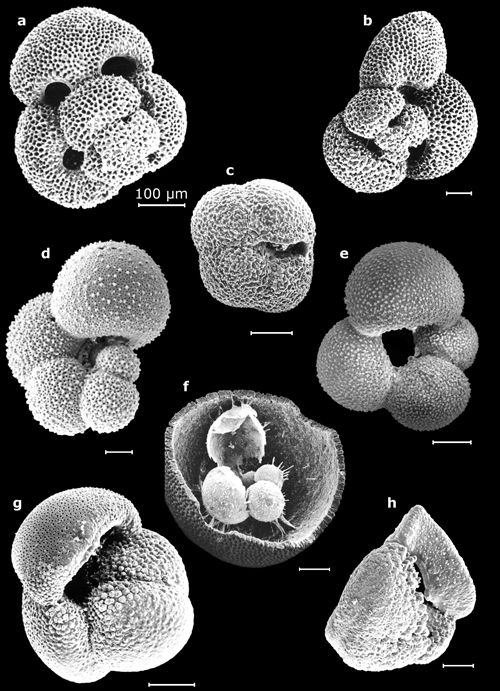
Click on the image to enlarge it.
Plate
6: SEM views of representative morphotypes of the genera Globigerinoides
(a: spiral view; b: spiral view in Mathieu, 1986,
Pl. 18, fig. 3), Neogloboquadrina
(c: umbilical view), Globigerinella (d: lateral view in Mathieu, 1986,
Pl. 18, fig. 5), Globigerina (e: umbilical view in Mathieu, 1986,
Pl. 18, fig. 7), Orbulina (f: expanded view in Mathieu, 1986,
Pl. 18, fig. 1), Globorotalia (g: umbilical view; h: profile in Mathieu, 1986,
Pl. 19, fig. 11) - Atlantic margin off Morocco. Graphical scales = 100 µm.
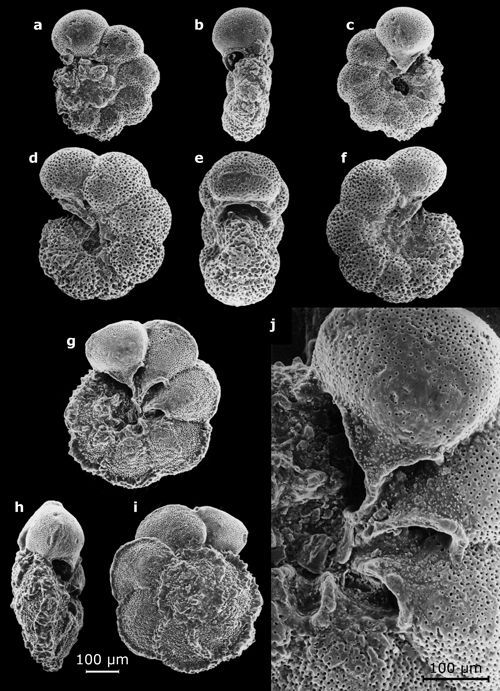
Click on the image to enlarge it.
Plate
7: SEM views of representative morphotypes of the genera Ticinella
(a: spiral face; b: profile; c: umbilical face), Biticinella
(d et f: lateral faces; e: profile), Rotalipora (g: spiral face; h:
profile; i: umbilical face; j: detail of g, main aperture and supplementary
apertures) - ODP Leg 171B, Site 1050. Graphical scales = 100 µm.
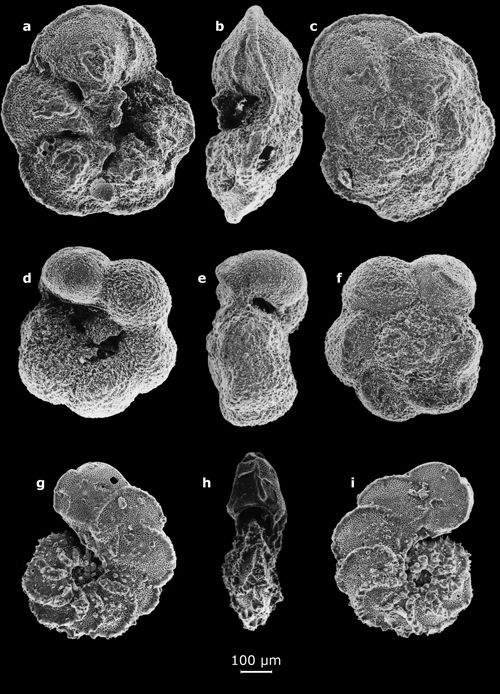
Click on the image to enlarge it.
Plate
8: SEM views of representative morphotypes of the genera Rotalipora
(a: umbilical face; b: profile; c: spiral face), Helvetoglobotruncana (d:
umbilical face; e: profile; f: spiral face), Planomalina (g and i:
lateral faces; h: profile) - ODP Leg 171B, Site 1050. Graphical scales = 100 µm.
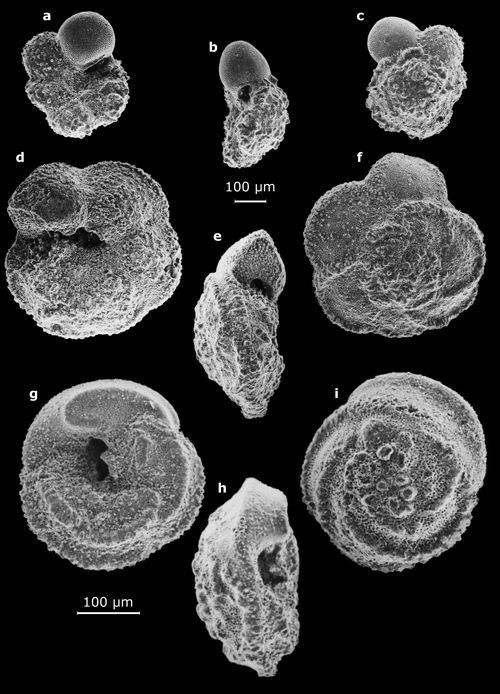
Click on the image to enlarge it.
Plate
9: SEM views of representative morphotypes of the genera Praeglobotruncana
(a: umbilical face; b: profile; c: spiral face), Dicarinella (d:
umbilical face; e: profile; f: spiral face), Contusotruncana (g:
umbilical face; h: profile; i: spiral face) - ODP Leg 171B, Site 1050. Graphical
scales = 100 µm.
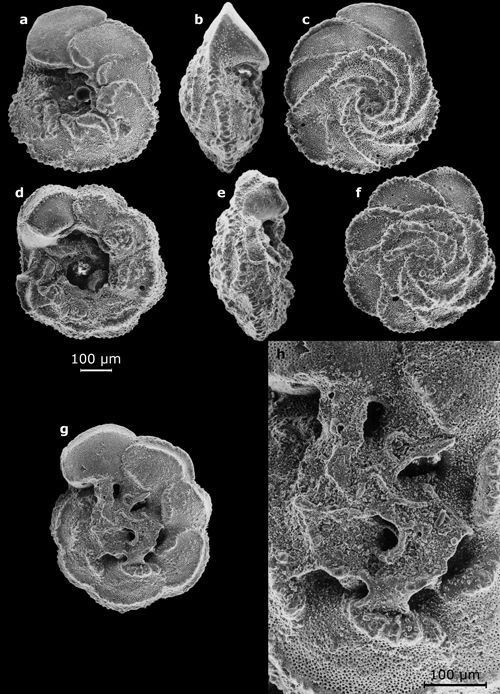
Click on the image to enlarge it.
Plate
10: SEM views of representative morphotypes of the genera Globotruncanita
(a: umbilical face; b: profile; c: spiral face), Globotruncana (d and
g: umbilical faces; e: profile; f: spiral face; h: detail of g, umbilical
plates) - ODP Leg 171B, Site 1050. Graphical scales = 100 µm.
Citation
Bellier J.-P., Mathieu R. & Granier B. (2010).-
Short Treatise on Foraminiferology (Essential on modern and fossil Foraminifera)
[Court traité de
foraminiférologie (L'essentiel sur les foraminifères actuels et fossiles)].-
Carnets
de Géologie - Notebooks on Geology, Brest, Book 2010/02 (CG2010_B02),
104 p., 15
figs, 10 pls.
 - 2
- 2  ). These are forms that live on the sea
floor, either on the surface of the sediment or buried in it (endofauna), or are attached to plant stems, rocks or particles
(epifauna);
). These are forms that live on the sea
floor, either on the surface of the sediment or buried in it (endofauna), or are attached to plant stems, rocks or particles
(epifauna); ).
These are forms which float passively, moved only by currents but capable of vertical migration.
).
These are forms which float passively, moved only by currents but capable of vertical migration.























