![]() The fossil renamed here was first described in
1959 as Aeolisaccus
kotori , a new species of a problematic fossil worm, Aeolisaccus
. In 1975 recognized the true
relationships of this microbial
fossil: a cyanobacterium related closely to the modern genus Scytonema. The
fossil is common in the sediments of the Mesozoic carbonate platforms of
southern Europe. This contribution confirmed 's interpretation,
determined, using the high resolution of the SEM, the extent to which these fossils have preserved their original architecture, and
investigated their presumed modern counterparts among the abundant mat-forming
species of Scytonema on the intertidal flats of Andros Island, a part of the Bahama carbonate platform. The systematic
affinities of the fossil and the environments it inhabited were reconstructed by
comparing the morphology of the fossils to that of their modern counterparts,
along with their respective sedimentary contexts. Based on these comparisons, we
conclude that the organism lived in a peritidal environment and was buried and
fossilized in the shallow waters of an ancient carbonate platform. A formal
transfer of the fossil to a new genus of fossil cyanobacteria thereby
designated as Decastronema gen. nov. is proposed, honoring the contribution of Prof.
Piero to paleontology.
The fossil renamed here was first described in
1959 as Aeolisaccus
kotori , a new species of a problematic fossil worm, Aeolisaccus
. In 1975 recognized the true
relationships of this microbial
fossil: a cyanobacterium related closely to the modern genus Scytonema. The
fossil is common in the sediments of the Mesozoic carbonate platforms of
southern Europe. This contribution confirmed 's interpretation,
determined, using the high resolution of the SEM, the extent to which these fossils have preserved their original architecture, and
investigated their presumed modern counterparts among the abundant mat-forming
species of Scytonema on the intertidal flats of Andros Island, a part of the Bahama carbonate platform. The systematic
affinities of the fossil and the environments it inhabited were reconstructed by
comparing the morphology of the fossils to that of their modern counterparts,
along with their respective sedimentary contexts. Based on these comparisons, we
conclude that the organism lived in a peritidal environment and was buried and
fossilized in the shallow waters of an ancient carbonate platform. A formal
transfer of the fossil to a new genus of fossil cyanobacteria thereby
designated as Decastronema gen. nov. is proposed, honoring the contribution of Prof.
Piero to paleontology.
![]() Aeolisaccus
kotori, Decastronema n. gen., carbonate platforms, cyanobacteria, diagenesis, microbial fossil, Cretaceous.
Aeolisaccus
kotori, Decastronema n. gen., carbonate platforms, cyanobacteria, diagenesis, microbial fossil, Cretaceous.
S., R. & L. (2006).- Decastronema kotori gen. nov., comb. nov.: a mat-forming cyanobacterium on Cretaceous carbonate platforms and its modern counterparts.- Carnets de Géologie / Notebooks on Geology, Brest, Article 2006/02 (CG2006_A02)
![]() Decastronema kotori
n. gen., n. comb., une cyanobactérie des tapis algaires des plates-formes carbonatées crétacées, et ses analogues modernes.-
Ce fossile fut décrit pour la première fois en 1959
sous le nom d'Aeolisaccus kotori , une nouvelle espèce
rapportée à un vers fossile énigmatique, Aeolisaccus
. En 1975,
reconnaissait la nature microbienne de ce fossile, une cyanobactérie très proche du genre actuel Scytonema.
Cet organisme est fréquemment observé dans les séries carbonatées des plates-formes
mésozoïques d'Europe méridionale. Le présent travail confirme
l'interprétation de . L'examen au MEB
montre la remarquabe préservation de l'architecture originelle de
ces fossiles et permet d'établir des rapprochements significatifs avec les
nombreuses espèces modernes de Scytonema participant à l'élaboration des tapis
algaires dans les zones d'estran de l'île d'Andros sur la plate-forme
carbonatée des Bahamas. L'établissement des affinités taxinomiques des fossiles et
la reconstitution des environnements qu'ils occupaient ont été effectués en comparant les
morphologies des fossiles et de leurs analogues actuels, et en prenant
également en considération leurs contextes sédimentaires respectifs. En
s'appuyant sur ces comparaisons, nous concluons que ces organismes vivaient dans
un milieu péritidal et qu'ils ont été enfouis, puis fossilisés
en eaux peu profondes sur une plate-forme carbonatée ancienne. Nous proposons
le transfert formel de ce fossile dans un nouveau genre de cyanobactéries
fossiles créé pour cette occasion et nommé Decastronema n. gen. en l'honneur du Professeur
Piero pour sa contribution à la paléontologie.
Decastronema kotori
n. gen., n. comb., une cyanobactérie des tapis algaires des plates-formes carbonatées crétacées, et ses analogues modernes.-
Ce fossile fut décrit pour la première fois en 1959
sous le nom d'Aeolisaccus kotori , une nouvelle espèce
rapportée à un vers fossile énigmatique, Aeolisaccus
. En 1975,
reconnaissait la nature microbienne de ce fossile, une cyanobactérie très proche du genre actuel Scytonema.
Cet organisme est fréquemment observé dans les séries carbonatées des plates-formes
mésozoïques d'Europe méridionale. Le présent travail confirme
l'interprétation de . L'examen au MEB
montre la remarquabe préservation de l'architecture originelle de
ces fossiles et permet d'établir des rapprochements significatifs avec les
nombreuses espèces modernes de Scytonema participant à l'élaboration des tapis
algaires dans les zones d'estran de l'île d'Andros sur la plate-forme
carbonatée des Bahamas. L'établissement des affinités taxinomiques des fossiles et
la reconstitution des environnements qu'ils occupaient ont été effectués en comparant les
morphologies des fossiles et de leurs analogues actuels, et en prenant
également en considération leurs contextes sédimentaires respectifs. En
s'appuyant sur ces comparaisons, nous concluons que ces organismes vivaient dans
un milieu péritidal et qu'ils ont été enfouis, puis fossilisés
en eaux peu profondes sur une plate-forme carbonatée ancienne. Nous proposons
le transfert formel de ce fossile dans un nouveau genre de cyanobactéries
fossiles créé pour cette occasion et nommé Decastronema n. gen. en l'honneur du Professeur
Piero pour sa contribution à la paléontologie.
![]() Aeolisaccus kotori, Decastronema n. gen., plates-formes carbonatées, cyanobactéries, diagénèse, fossile microbien, Crétacé.
Aeolisaccus kotori, Decastronema n. gen., plates-formes carbonatées, cyanobactéries, diagénèse, fossile microbien, Crétacé.
![]() Decastronema kotori
gen. nov., comb. nov.,
cijanobakterija mikrobijskih pokrova na karbonatnim platformama Krede i njeni
recentni predstavnici.- Fosil, koji proučavamo
je izvorno je opisan 1959. godine pod imenom Aeolisaccus
kotori , kao nova vrsta
problematičnog fosila, Aeolisaccus .
revidira sistematsku pripadnost tog fosila 1975. godine i priključuje
ga u skupinu cijanobakterija srodnu recentnom rodu Scytonema. Fosil je
obilno zastupljen u sedimentima mesozojskih karbonatnih platforma južne Europe.
Ovim je radom potvrđena sistematska pripadnost fosila po ,
i to primjenom elektronske mikroskopije (SEM) u proučavanju njegove gradje. Nadalje se
istražuju mogući recentni srodnici među vrstama roda Scytonema,
koje grade mikrobijske pokrove u zoni plime i oseke na otoku Androsu (dio
Bahamske karbonatne platforme). Sistematska pripadnost i sredina u kojoj je
mezozojski fosil živio je rekonstruirana
na osnovi usporedbe morfologije fosilnih i recentih oblika u okviru
sedimentarnog konteksta. Na toj osnovi zaključujemo, da je organizam
obitavao u mediolitoralnoj zoni, te da je prenesen, odložen i sačuvan u
talozima plitkog mora drevne karbonatne platforme. Predlažemo formalni prijenos
fosila k novom rodu fosilnih cijanobakterija pod nazivom Decastronema gen. nov. a u
čast profesoru Pieru
za njegove znanstvene doprinose u području paleontologije.
Decastronema kotori
gen. nov., comb. nov.,
cijanobakterija mikrobijskih pokrova na karbonatnim platformama Krede i njeni
recentni predstavnici.- Fosil, koji proučavamo
je izvorno je opisan 1959. godine pod imenom Aeolisaccus
kotori , kao nova vrsta
problematičnog fosila, Aeolisaccus .
revidira sistematsku pripadnost tog fosila 1975. godine i priključuje
ga u skupinu cijanobakterija srodnu recentnom rodu Scytonema. Fosil je
obilno zastupljen u sedimentima mesozojskih karbonatnih platforma južne Europe.
Ovim je radom potvrđena sistematska pripadnost fosila po ,
i to primjenom elektronske mikroskopije (SEM) u proučavanju njegove gradje. Nadalje se
istražuju mogući recentni srodnici među vrstama roda Scytonema,
koje grade mikrobijske pokrove u zoni plime i oseke na otoku Androsu (dio
Bahamske karbonatne platforme). Sistematska pripadnost i sredina u kojoj je
mezozojski fosil živio je rekonstruirana
na osnovi usporedbe morfologije fosilnih i recentih oblika u okviru
sedimentarnog konteksta. Na toj osnovi zaključujemo, da je organizam
obitavao u mediolitoralnoj zoni, te da je prenesen, odložen i sačuvan u
talozima plitkog mora drevne karbonatne platforme. Predlažemo formalni prijenos
fosila k novom rodu fosilnih cijanobakterija pod nazivom Decastronema gen. nov. a u
čast profesoru Pieru
za njegove znanstvene doprinose u području paleontologije.
![]() Aeolisaccus kotori, Decastronema
gen. n., karbonatne platforme, cijanobakterije, diageneza, microbijski fossil,
Kreda.
Aeolisaccus kotori, Decastronema
gen. n., karbonatne platforme, cijanobakterije, diageneza, microbijski fossil,
Kreda.
![]() The fossil record of cyanobacteria is rich throughout more than 2000 Ma of Proterozoic time ( & ,
1992), but is comparatively poor for the 543 Ma of the Phanerozoic Era. Many Proterozoic microfossils are preserved in early diagenetic silica deposits. In contrast, most fossils of algae and cyanobacteria recovered from Phanerozoic strata are preserved in carbonates. Many of them were calcified during or immediately after their lifetime, which facilitated their preservation (,
1991; , 1991).
The fossil record of cyanobacteria is rich throughout more than 2000 Ma of Proterozoic time ( & ,
1992), but is comparatively poor for the 543 Ma of the Phanerozoic Era. Many Proterozoic microfossils are preserved in early diagenetic silica deposits. In contrast, most fossils of algae and cyanobacteria recovered from Phanerozoic strata are preserved in carbonates. Many of them were calcified during or immediately after their lifetime, which facilitated their preservation (,
1991; , 1991).
![]() Many Proterozoic cyanobacterial microfossils have morphotypically close modern counterparts, thus testifying to
an early evolutionary origin, early ecological specialization and conservative maintenance of the basic adaptive properties of this group of the original oxygenic phototrophs ( et alii,
1986; & ,
1992).
Many Proterozoic cyanobacterial microfossils have morphotypically close modern counterparts, thus testifying to
an early evolutionary origin, early ecological specialization and conservative maintenance of the basic adaptive properties of this group of the original oxygenic phototrophs ( et alii,
1986; & ,
1992).
![]() This contribution examines a microfossil common in southern Europe in the Cretaceous strata of Periadriatic carbonate platforms, which are sedimentologically comparable with the modern carbonate platforms of Florida and the Bahamas (see ,
1970; et alii,
1975; &
,
1999; et alii,
2002). The fossil was originally described as Aeolisaccus kotori and attributed to the problematic fossil worm: Aeolisaccus
1958 ( 1959). Based on a detailed study of morphology and the morphometric evaluation of a large fossil population,
(1975) provided a convincing re-interpretation of this fossil as the remains of an ancient cyanobacterium. In support of his interpretation, invoked possible modern counterparts among mat-forming cyanobacteria, described by
(1967) from the mudflats on Andros Island, Bahamas. Here we investigate the state of preservation of this fossil using high resolution SEM imaging to compare it with the modern mat-forming Scytonema populations of Andros Island, Bahamas. Based on confirmed cyanobacterial identity, and in accordance with the rules of zoological nomenclature, we propose to change the name of this fossil.
This contribution examines a microfossil common in southern Europe in the Cretaceous strata of Periadriatic carbonate platforms, which are sedimentologically comparable with the modern carbonate platforms of Florida and the Bahamas (see ,
1970; et alii,
1975; &
,
1999; et alii,
2002). The fossil was originally described as Aeolisaccus kotori and attributed to the problematic fossil worm: Aeolisaccus
1958 ( 1959). Based on a detailed study of morphology and the morphometric evaluation of a large fossil population,
(1975) provided a convincing re-interpretation of this fossil as the remains of an ancient cyanobacterium. In support of his interpretation, invoked possible modern counterparts among mat-forming cyanobacteria, described by
(1967) from the mudflats on Andros Island, Bahamas. Here we investigate the state of preservation of this fossil using high resolution SEM imaging to compare it with the modern mat-forming Scytonema populations of Andros Island, Bahamas. Based on confirmed cyanobacterial identity, and in accordance with the rules of zoological nomenclature, we propose to change the name of this fossil.
![]() R. collected the fossiliferous rocks for this study in the Upper Cretaceous outcrop of
Mt. Grebnik, Mirdita Zone, Serbia and Montenegro
(Fig. 1
R. collected the fossiliferous rocks for this study in the Upper Cretaceous outcrop of
Mt. Grebnik, Mirdita Zone, Serbia and Montenegro
(Fig. 1 ![]() ). Fossil cyanobacterial fragments occur in great numbers in the lens-shaped deposits of fine-grained mudstone, wackestone and packstone immediately above paleokarstic bauxite deposits
(Fig. 2
). Fossil cyanobacterial fragments occur in great numbers in the lens-shaped deposits of fine-grained mudstone, wackestone and packstone immediately above paleokarstic bauxite deposits
(Fig. 2 ![]() ). More were obtained from the collection of P. , University of Naples, Italy. S. collected modern specimens of Scytonema on the mud flats (with ponds and mangroves) on the west coast of Andros Island, Bahamas, mainly in the area bounded by the tidal creeks Polamasola and Three Creeks. The collected specimens were preserved both air-dried and in solution of 3% formaldehyde in environmental water.
). More were obtained from the collection of P. , University of Naples, Italy. S. collected modern specimens of Scytonema on the mud flats (with ponds and mangroves) on the west coast of Andros Island, Bahamas, mainly in the area bounded by the tidal creeks Polamasola and Three Creeks. The collected specimens were preserved both air-dried and in solution of 3% formaldehyde in environmental water.
![]() Petrographic thin sections were prepared from compact fine-grained Cretaceous limestone cut both perpendicular to and parallel with to the bedding plane. They were examined using both transmitted and cross-polarized light microscopy. Other sections were subsequently polished, briefly etched with dilute HCl, washed in deionized water, dried, coated with gold-palladium and studied using scanning electron microscopy.
Petrographic thin sections were prepared from compact fine-grained Cretaceous limestone cut both perpendicular to and parallel with to the bedding plane. They were examined using both transmitted and cross-polarized light microscopy. Other sections were subsequently polished, briefly etched with dilute HCl, washed in deionized water, dried, coated with gold-palladium and studied using scanning electron microscopy.
![]() Specimens of modern Scytonema were washed in deionized water prior to analysis and mounted on slides for transmitted light microscopy. Selected samples were gradually dehydrated in an ethanol series and critical-point-dried using liquid CO2. Whole and fragmented specimens were observed by SEM.
Specimens of modern Scytonema were washed in deionized water prior to analysis and mounted on slides for transmitted light microscopy. Selected samples were gradually dehydrated in an ethanol series and critical-point-dried using liquid CO2. Whole and fragmented specimens were observed by SEM.
![]() The fossil was originally described (,
1959) as minute thick-walled cylindrical bags closed at one end, ca 500 to 780 µm long, with 32 to 80 µm external and 10 to 24 µm internal
diameters. It was ascribed to the genus Aeolisaccus
1958, under the name A. kotori
1959. A supplementary description with photomicrographs was published later (,
1972) showing calcareous tubes with thick walls composed of a series of inserted conical units.
(1975) examined and measured large populations of this fossil in shallow-water Senonian carbonates from the Apenninic Carbonate Platform. He recognized that the the peculiar architecture of the walls was comparable to that of modern scytonematacean cyanobacteria and deduced that this fossil was an ancient cyanobacterium.
The fossil was originally described (,
1959) as minute thick-walled cylindrical bags closed at one end, ca 500 to 780 µm long, with 32 to 80 µm external and 10 to 24 µm internal
diameters. It was ascribed to the genus Aeolisaccus
1958, under the name A. kotori
1959. A supplementary description with photomicrographs was published later (,
1972) showing calcareous tubes with thick walls composed of a series of inserted conical units.
(1975) examined and measured large populations of this fossil in shallow-water Senonian carbonates from the Apenninic Carbonate Platform. He recognized that the the peculiar architecture of the walls was comparable to that of modern scytonematacean cyanobacteria and deduced that this fossil was an ancient cyanobacterium.
![]() When observed in petrographic thin sections under transmitted
light the fossils appear as cylindrical tubules, each characterized by a dark wall and a clear lumen. The walls are cylindrical and smooth internally, but uneven externally
(Fig. 3A
When observed in petrographic thin sections under transmitted
light the fossils appear as cylindrical tubules, each characterized by a dark wall and a clear lumen. The walls are cylindrical and smooth internally, but uneven externally
(Fig. 3A ![]() ). They are composed of funnels that diverge outward, and often have thin and undulating margins
(Fig. 3B
). They are composed of funnels that diverge outward, and often have thin and undulating margins
(Fig. 3B ![]() ). This unique architecture can be examined by optical sectioning, or reconstructed from numerous transverse, oblique and longitudinal cuts through the fossils in thin section. The three-dimensional aspect of the series of inserted funnels is deducible from transitions between saggital and tangential sections
(Figs. 3C-E
). This unique architecture can be examined by optical sectioning, or reconstructed from numerous transverse, oblique and longitudinal cuts through the fossils in thin section. The three-dimensional aspect of the series of inserted funnels is deducible from transitions between saggital and tangential sections
(Figs. 3C-E ![]() ). The fossil tubules, of different lengths and randomly oriented, are scattered in the sediment in densities ranging up to 100 tubules
mm3. Such a distribution suggests that they were deposited as fragments, possibly after some transportation. They were never seen in growth position.
). The fossil tubules, of different lengths and randomly oriented, are scattered in the sediment in densities ranging up to 100 tubules
mm3. Such a distribution suggests that they were deposited as fragments, possibly after some transportation. They were never seen in growth position.
![]() Longer tubules are sometimes branched, that is the clear central core (lumen) along with the inner layers of the wall penetrates laterally through the outer wall to form a branch. Several cases of this unusual
'inside out' branching pattern were seen and illustrated by
(1975, Pl. 5, figs. 1-9), who recognized them as false branching of the type characteristic of the filaments in the family Scytonemataceae.
Longer tubules are sometimes branched, that is the clear central core (lumen) along with the inner layers of the wall penetrates laterally through the outer wall to form a branch. Several cases of this unusual
'inside out' branching pattern were seen and illustrated by
(1975, Pl. 5, figs. 1-9), who recognized them as false branching of the type characteristic of the filaments in the family Scytonemataceae.
![]() The SEM images of polished and lightly etched preparations that expose the fossil structures in low relief show morphological properties the same as those visible in light microscopy
(Fig. 4A
The SEM images of polished and lightly etched preparations that expose the fossil structures in low relief show morphological properties the same as those visible in light microscopy
(Fig. 4A ![]() ). At a higher resolution the fossils appear to be constructed mainly of minerals. At magnifications ranging from 300 to 4000x the SEM images indicate that preservation of wall structure was accomplished in two ways.
). At a higher resolution the fossils appear to be constructed mainly of minerals. At magnifications ranging from 300 to 4000x the SEM images indicate that preservation of wall structure was accomplished in two ways.
![]() In the more common mode of preservation, the fossil structure is expressed entirely by mineral grains (crystallites) with sharp euhedral boundaries
(Figs. 4B-C
In the more common mode of preservation, the fossil structure is expressed entirely by mineral grains (crystallites) with sharp euhedral boundaries
(Figs. 4B-C ![]() ). The walls of the tubules are comprised of very fine micrite with isodiametric grains ranging between 0.3 and 0.8 µm in diameter. The layers of the wall are made up of alternating relatively coarser (0.4-0.8 µm) and finer (0.3-0.5 µm) grains of micrite. Each layer is characterized by a different and uniform grain size of micrite with sharp euhedral boundaries
(Fig. 4G
). The walls of the tubules are comprised of very fine micrite with isodiametric grains ranging between 0.3 and 0.8 µm in diameter. The layers of the wall are made up of alternating relatively coarser (0.4-0.8 µm) and finer (0.3-0.5 µm) grains of micrite. Each layer is characterized by a different and uniform grain size of micrite with sharp euhedral boundaries
(Fig. 4G ![]() ). In cross sections of the filaments the layers are concentric
(Fig. 4B
). In cross sections of the filaments the layers are concentric
(Fig. 4B ![]() ), in longitudinal sections V-shaped
(Fig. 4C
), in longitudinal sections V-shaped
(Fig. 4C ![]() ). The lumen of the tubules is filled with larger grains 10-15 µm in diameter.
). The lumen of the tubules is filled with larger grains 10-15 µm in diameter.
![]() The second mode of preservation was found in specimens with walls of a reddish, rust-color when viewed with an incident light microscope. Under SEM, these walls evince divergent layers
(Figs. 4A & D-F
The second mode of preservation was found in specimens with walls of a reddish, rust-color when viewed with an incident light microscope. Under SEM, these walls evince divergent layers
(Figs. 4A & D-F ![]() ) like those of the other mode.
At higher magnifications the walls are not granular, but spongy with a fine, submicron level porosity
(Fig. 4H
) like those of the other mode.
At higher magnifications the walls are not granular, but spongy with a fine, submicron level porosity
(Fig. 4H ![]() ). The reddish color is due to ferric oxides and hydroxides (limonite). The wall structure in these specimens is less affected by acid so it produces deeper relief as, for example, the conical shape of the layers in the three-dimensional display
(Fig. 4I
). The reddish color is due to ferric oxides and hydroxides (limonite). The wall structure in these specimens is less affected by acid so it produces deeper relief as, for example, the conical shape of the layers in the three-dimensional display
(Fig. 4I ![]() ).
).
![]() In both types of preservation, the lumen of the tubules is filled with relatively large micritic and microsparitic grains (cf.
Figs. 4B & D
In both types of preservation, the lumen of the tubules is filled with relatively large micritic and microsparitic grains (cf.
Figs. 4B & D ![]() ), up to 20 µm wide and 40 µm long, commonly conforming to the internal diameter of the tubule
(Fig. 4E
), up to 20 µm wide and 40 µm long, commonly conforming to the internal diameter of the tubule
(Fig. 4E ![]() ). The clarity of the lumen observed in thin sections by light microscopy
(Fig. 3
). The clarity of the lumen observed in thin sections by light microscopy
(Fig. 3 ![]() )
is explained by the greater conductance of light in the relatively large calcitic grains that occupy the lumen
(Figs. 4E-F
)
is explained by the greater conductance of light in the relatively large calcitic grains that occupy the lumen
(Figs. 4E-F ![]() ). Their large size suggests that the tubes were empty when
the grains were deposited. The clear interiors of the tubes are in marked contrast to the dark appearance of the
walls which is caused by light diffraction and scattering in the numerous small crystals.
). Their large size suggests that the tubes were empty when
the grains were deposited. The clear interiors of the tubes are in marked contrast to the dark appearance of the
walls which is caused by light diffraction and scattering in the numerous small crystals.
![]() The two modes of preservation are indistinguishable under transmitted light microscopy. Therefore, it is unlikely that the iron accumulated during the lifetime of the organism. The wall may have been enriched in iron postdepositionally by chelation with the organic matter associated with the sheath. Ferrous iron in solution may have leached from the nearby bauxite deposits, then transported to overlying beds in anoxic ground water and later oxidized to limonite.
The two modes of preservation are indistinguishable under transmitted light microscopy. Therefore, it is unlikely that the iron accumulated during the lifetime of the organism. The wall may have been enriched in iron postdepositionally by chelation with the organic matter associated with the sheath. Ferrous iron in solution may have leached from the nearby bauxite deposits, then transported to overlying beds in anoxic ground water and later oxidized to limonite.
![]() The carbonate matrix surrounding the fossils is an irregular network of unsorted carbonate grains of different sizes with streaks of finer grains meandering around islets of larger sparitic grains
(Figs. 4A & F
The carbonate matrix surrounding the fossils is an irregular network of unsorted carbonate grains of different sizes with streaks of finer grains meandering around islets of larger sparitic grains
(Figs. 4A & F ![]() ). The size of the grains comprising these islets often show a progressive centripetal increase, a characteristic of pore fills. This suggests that the fossil was deposited in a fine-grained porous sediment (possibly calcareous mud) and later diagenetically altered by cementation and pore filling.
The mudstone - wackestone matrix of the fossils presumably
was laid down in an oxidized low-energy shallow marine environment on an ancient
carbonate platform. This interpretation is consistent with the lack of preserved organic matter and the absence of reduced minerals.
). The size of the grains comprising these islets often show a progressive centripetal increase, a characteristic of pore fills. This suggests that the fossil was deposited in a fine-grained porous sediment (possibly calcareous mud) and later diagenetically altered by cementation and pore filling.
The mudstone - wackestone matrix of the fossils presumably
was laid down in an oxidized low-energy shallow marine environment on an ancient
carbonate platform. This interpretation is consistent with the lack of preserved organic matter and the absence of reduced minerals.
![]() The abundance of these fossils ranges widely. They may have been distributed over a large area of shallow sea or accumulated in minor depressions on a mud flat. The site in the Mirdita zone of Metohija contains high concentration of fossils in a lens-shaped accumulation. Its position immediately above terrigenous bauxite deposits suggests early transgression. We assume that prior to deposition the fossils had been transported by tidal currents.
The abundance of these fossils ranges widely. They may have been distributed over a large area of shallow sea or accumulated in minor depressions on a mud flat. The site in the Mirdita zone of Metohija contains high concentration of fossils in a lens-shaped accumulation. Its position immediately above terrigenous bauxite deposits suggests early transgression. We assume that prior to deposition the fossils had been transported by tidal currents.
![]() (1975) recognized the unique architecture of the fossil, specifically the size, false branching and divergent layering of the wall, as characteristic of cyanobacterial sheaths. In search of the biological affinity of the fossil organism and its possible modern counterpart, referred to 's
(1967) study of modern microbial mats on Andros Island, Bahamas and considered among others a mat-forming cyanobacterium identified as Scytonema myochrous. Earlier,
(1933) had described from that island two species with similar characteristics, but from a predominantly freshwater habitats: Scytonema crustaceum and S. androsense. However,
thought the fossil more closely related to the modern genus Tolypothrix because of the prevalence of single-sided false branching
(1975) recognized the unique architecture of the fossil, specifically the size, false branching and divergent layering of the wall, as characteristic of cyanobacterial sheaths. In search of the biological affinity of the fossil organism and its possible modern counterpart, referred to 's
(1967) study of modern microbial mats on Andros Island, Bahamas and considered among others a mat-forming cyanobacterium identified as Scytonema myochrous. Earlier,
(1933) had described from that island two species with similar characteristics, but from a predominantly freshwater habitats: Scytonema crustaceum and S. androsense. However,
thought the fossil more closely related to the modern genus Tolypothrix because of the prevalence of single-sided false branching
![]() We have studied the modern mat-building cyanobacteria on the mud flats of the west coast of Andros Island, which is influenced by marine tidal circulation. Two species of Scytonema are common there. One inhabits a narrow range of the intertidal zone on the seaward side of the barrier beach, whereas the other covers vast surfaces around mangrove bushes on the ponded mud flats behind the barrier beach. The morphology of the mud-flat-dwelling species of Scytonema is closer to that of the Cretaceous fossil. The similarities include divergent sheath layering as well as the prevalence of single sided false branching, which like that of Tolypothrix is related to its upright growth
(Fig. 5A
We have studied the modern mat-building cyanobacteria on the mud flats of the west coast of Andros Island, which is influenced by marine tidal circulation. Two species of Scytonema are common there. One inhabits a narrow range of the intertidal zone on the seaward side of the barrier beach, whereas the other covers vast surfaces around mangrove bushes on the ponded mud flats behind the barrier beach. The morphology of the mud-flat-dwelling species of Scytonema is closer to that of the Cretaceous fossil. The similarities include divergent sheath layering as well as the prevalence of single sided false branching, which like that of Tolypothrix is related to its upright growth
(Fig. 5A ![]() ). The filaments consist of thick sheaths harboring much narrower cellular trichomes in their core. The outer margins of V-shaped layers show externally as barely visible rings. The relationship between cellular trichome and layered sheath visible in fractured specimens
(Figs. 5B-C
). The filaments consist of thick sheaths harboring much narrower cellular trichomes in their core. The outer margins of V-shaped layers show externally as barely visible rings. The relationship between cellular trichome and layered sheath visible in fractured specimens
(Figs. 5B-C ![]() )
is similar to the architecture of the walls of the Cretaceous fossil (compare
Fig. 4I
)
is similar to the architecture of the walls of the Cretaceous fossil (compare
Fig. 4I ![]() ).
).
![]() Thick envelopes and sheaths produced by copious amounts of extracellular polymeric substances (EPS) are common, particularly in subaerially growing cyanobacteria. However, the characteristic sheath architecture with open, upwardly divergent layering is particular to the section Myochrotes within the genus Scytonema (
et 1887). Trichomes of Scytonema have the highest rates of cell division and sheath excretion at their tips, thus forming apical meristems; both decrease basipetally as the cells grow older. The newly excreted portion of the sheath encloses several actively growing cells below the apex
(Fig. 6A
Thick envelopes and sheaths produced by copious amounts of extracellular polymeric substances (EPS) are common, particularly in subaerially growing cyanobacteria. However, the characteristic sheath architecture with open, upwardly divergent layering is particular to the section Myochrotes within the genus Scytonema (
et 1887). Trichomes of Scytonema have the highest rates of cell division and sheath excretion at their tips, thus forming apical meristems; both decrease basipetally as the cells grow older. The newly excreted portion of the sheath encloses several actively growing cells below the apex
(Fig. 6A ![]() ). Due to apical growth, cell division and the accompanying production of EPS move in an acropetal direction. Consequently, the envelopes assume the asymmetrical shape of inverted cones
(Fig. 6B
). Due to apical growth, cell division and the accompanying production of EPS move in an acropetal direction. Consequently, the envelopes assume the asymmetrical shape of inverted cones
(Fig. 6B ![]() ).
The angle of these cones is a function of the rate at which the trichome elongates: it is more divergent when the growth is slow, and almost parallel in fast-growing aquatic forms (,
1934). The pressure of the growing trichomes causes consecutive bursting and opening of envelopes at the trichome tip so the continuing growth produces a series of characteristic, funnel-shaped collars
(Fig. 7
).
The angle of these cones is a function of the rate at which the trichome elongates: it is more divergent when the growth is slow, and almost parallel in fast-growing aquatic forms (,
1934). The pressure of the growing trichomes causes consecutive bursting and opening of envelopes at the trichome tip so the continuing growth produces a series of characteristic, funnel-shaped collars
(Fig. 7 ![]() , below). This feature has a potentiality for preservation and thus for recognition in the fossil record (see & ,
1976). In addition, nitrogen fixation in these organisms, performed by specialized cells called heterocysts, stimulates growth in the middle of the trichome, forming intercalary meristems. Differentiated heterocysts do not produce EPS and act as anchoring points for trichomes, restricting their gliding inside the sheaths ( et alii,
1996). As a consequence, the trichomes protrude through the old sheath and continue to grow as false branches.
, below). This feature has a potentiality for preservation and thus for recognition in the fossil record (see & ,
1976). In addition, nitrogen fixation in these organisms, performed by specialized cells called heterocysts, stimulates growth in the middle of the trichome, forming intercalary meristems. Differentiated heterocysts do not produce EPS and act as anchoring points for trichomes, restricting their gliding inside the sheaths ( et alii,
1996). As a consequence, the trichomes protrude through the old sheath and continue to grow as false branches.
![]() The presence of these same properties in the fossil Decastronema filaments, in particular the collar-like opening of sheath layers in conjunction with false branching, as well as an overall similarity in shape and size demonstrates unquestionably that 's interpretation and identification of this organism as an ancient cyanobacterium is valid.
The presence of these same properties in the fossil Decastronema filaments, in particular the collar-like opening of sheath layers in conjunction with false branching, as well as an overall similarity in shape and size demonstrates unquestionably that 's interpretation and identification of this organism as an ancient cyanobacterium is valid.
![]() Using light microscopy,
(1975) saw in longitudinal sections of Decastronema tubules, faint dark lines across the lumen. He interpreted these lines as the possible cross walls of the cellular trichomes, which would imply cell lengths of up to 30 µm. Our use of cross-polarized light microscopy and SEM images resolved these lines as grain boundaries between microsparitic grains that filled the tubes, often in a single row
(Figs. 4D-E
Using light microscopy,
(1975) saw in longitudinal sections of Decastronema tubules, faint dark lines across the lumen. He interpreted these lines as the possible cross walls of the cellular trichomes, which would imply cell lengths of up to 30 µm. Our use of cross-polarized light microscopy and SEM images resolved these lines as grain boundaries between microsparitic grains that filled the tubes, often in a single row
(Figs. 4D-E ![]() ). At the level of resolution used in this
study no remains of trichome cells or cross walls separating them were found.
). At the level of resolution used in this
study no remains of trichome cells or cross walls separating them were found.
![]() The meristematic (i.e. rapidly dividing) cells in modern Scytonema are short and wide. As the rate of division slows, the cells grow longer and narrower, accompanied by a loss in turgescence. The consequence is a wide variability in the dimensions of the cells, which is consistent with the considerable variability of the internal diameters of Decastronema tubules.
The meristematic (i.e. rapidly dividing) cells in modern Scytonema are short and wide. As the rate of division slows, the cells grow longer and narrower, accompanied by a loss in turgescence. The consequence is a wide variability in the dimensions of the cells, which is consistent with the considerable variability of the internal diameters of Decastronema tubules.
![]() The appearance of one-sided
'closure' reported in the original description (,
1959) exists also in modern Scytonema, but is limited to growing tips and young false branches. This condition is explained by the life cycle and mode of reproduction of these filamentous cyanobacteria, during which trichomes fragment to produce and release short segments called hormogonia, which then open and leave the sheath. A similar feature was described as a
'terminal chamber' in Decastronema and interpreted as a possible heterocyst (,
1975, Pl. 6, Fig. 1). Actually, it represents a section through a curved or falsely branched filament of which a part was sectioned longitudinally and the other part transversally
(Fig. 3F
The appearance of one-sided
'closure' reported in the original description (,
1959) exists also in modern Scytonema, but is limited to growing tips and young false branches. This condition is explained by the life cycle and mode of reproduction of these filamentous cyanobacteria, during which trichomes fragment to produce and release short segments called hormogonia, which then open and leave the sheath. A similar feature was described as a
'terminal chamber' in Decastronema and interpreted as a possible heterocyst (,
1975, Pl. 6, Fig. 1). Actually, it represents a section through a curved or falsely branched filament of which a part was sectioned longitudinally and the other part transversally
(Fig. 3F ![]() ).
).
![]() The habitat of the coastal
Scytonema of Andros Island is an intertidal mudflat, flooded by meandering tidal creeks and periodically flushed by seawater during storms. Freshwater comes from rain and the drainage of water. Thus, salinity fluctuates from brackish to hypersaline. The organisms endure periodic shortages of water and an excessive solar illumination. Their thick sheaths may slow down the loss of water; they also contain the dark pigment scytonemin
which provides a modicum of protection against damage from excessive light and UV radiation (,
1998).
The habitat of the coastal
Scytonema of Andros Island is an intertidal mudflat, flooded by meandering tidal creeks and periodically flushed by seawater during storms. Freshwater comes from rain and the drainage of water. Thus, salinity fluctuates from brackish to hypersaline. The organisms endure periodic shortages of water and an excessive solar illumination. Their thick sheaths may slow down the loss of water; they also contain the dark pigment scytonemin
which provides a modicum of protection against damage from excessive light and UV radiation (,
1998).
![]() We consider this organism to be the closest modern counterpart of
Decastronema. The fossil must have had ecological requirements very much like those of the modern species. Therefore we propose a similar habitat for the Cretaceous form.
We consider this organism to be the closest modern counterpart of
Decastronema. The fossil must have had ecological requirements very much like those of the modern species. Therefore we propose a similar habitat for the Cretaceous form.
![]() In 1975
determined that the microfossil he was studying was misidentified and that its true taxonomic affinity is among the cyanobacteria. This called for a new nomenclatural combination with an assignment to a different genus. However, in view of the remaining uncertainties in the identification of the fossil, hesitated
"to transfer the species Aeolisaccus kotori from a 'genus' to which it certainly does not belong to another to which it might not belong."
(...) "For the moment," he concluded, "it seemed preferable to indicate the species under study by the name assigned to it by , at least until further information permits to specify the existing doubts".
In 1975
determined that the microfossil he was studying was misidentified and that its true taxonomic affinity is among the cyanobacteria. This called for a new nomenclatural combination with an assignment to a different genus. However, in view of the remaining uncertainties in the identification of the fossil, hesitated
"to transfer the species Aeolisaccus kotori from a 'genus' to which it certainly does not belong to another to which it might not belong."
(...) "For the moment," he concluded, "it seemed preferable to indicate the species under study by the name assigned to it by , at least until further information permits to specify the existing doubts".
![]() Here we confirm 's interpretation of Aeolisaccus kotori
as fossilized sheaths of an ancient scytonematacean cyanobacterium. Our detailed investigations give us added confidence, so we propose a separate generic identity for this now well-defined microbial fossil, placing it in the Phylum (Division) Cyanobacteria, Order Nostocales, Family Scytonemataceae as:
Here we confirm 's interpretation of Aeolisaccus kotori
as fossilized sheaths of an ancient scytonematacean cyanobacterium. Our detailed investigations give us added confidence, so we propose a separate generic identity for this now well-defined microbial fossil, placing it in the Phylum (Division) Cyanobacteria, Order Nostocales, Family Scytonemataceae as:
![]() Etymology: In honor of Professor Piero , of the University of Naples, Italy, for his valuable contributions to paleontology.
Etymology: In honor of Professor Piero , of the University of Naples, Italy, for his valuable contributions to paleontology.
![]() Diagnosis: Tubular filaments with bright core and dark walls, comprised of divergent and externally tapering layers arranged like a stack of
inserted cones. The layers appear concentric in transverse and V-shaped in longitudinal section.
Diagnosis: Tubular filaments with bright core and dark walls, comprised of divergent and externally tapering layers arranged like a stack of
inserted cones. The layers appear concentric in transverse and V-shaped in longitudinal section.
![]() Iconotype:
Figs. 3B-C & E
Iconotype:
Figs. 3B-C & E ![]()
![]() Description: Filaments and filament fragments with bright lumen and dark walls (as observed in transmitted light in petrographic thin sections), preserved mostly as tubules with walls comprised of divergent and externally tapering layers
(Figs. 3A-F
Description: Filaments and filament fragments with bright lumen and dark walls (as observed in transmitted light in petrographic thin sections), preserved mostly as tubules with walls comprised of divergent and externally tapering layers
(Figs. 3A-F ![]() ). The remains of the walls are preserved in a distinctive grain arrangement
(Figs. 4B-C & G
). The remains of the walls are preserved in a distinctive grain arrangement
(Figs. 4B-C & G ![]() ) or by iron-rich, spongy textures
(Figs. 4A, D-F & H-I
) or by iron-rich, spongy textures
(Figs. 4A, D-F & H-I ![]() ), rust-colored in polished slabs and petrographic thin sections. The tubules of D. kotori have an internal diameter of 14.2 ± 4.05 (330) µm expressed as Mean ± Standard Deviation (n). The walls appear dark and thick in petrographic thin sections, with an external diameter averaging 55.6 ± 12.5 (330) µm (recalculated after ,
1975), characterized by divergent layers that in three dimensions form as a stack of
inserted funnels. The outer margins of the divergent layers are often thinned and curved
(Figs. 3A-C
), rust-colored in polished slabs and petrographic thin sections. The tubules of D. kotori have an internal diameter of 14.2 ± 4.05 (330) µm expressed as Mean ± Standard Deviation (n). The walls appear dark and thick in petrographic thin sections, with an external diameter averaging 55.6 ± 12.5 (330) µm (recalculated after ,
1975), characterized by divergent layers that in three dimensions form as a stack of
inserted funnels. The outer margins of the divergent layers are often thinned and curved
(Figs. 3A-C ![]() ). Most tubules are short, randomly distributed fragments open at both ends. Larger fragments are often branched, which in well-preserved specimens can be recognized as being one-sided false branches -- the core and inner layers of the wall protrude through a lateral break and perforate the outer layer of the main filament to form a branch.
). Most tubules are short, randomly distributed fragments open at both ends. Larger fragments are often branched, which in well-preserved specimens can be recognized as being one-sided false branches -- the core and inner layers of the wall protrude through a lateral break and perforate the outer layer of the main filament to form a branch.
![]() Decastronema kotori is interpreted as the fossil remains of a large filamentous microorganism, which produced and dwelt in tubular sheaths made of extracellular polymeric substances (EPS, polysaccharides with some polypeptides). The architecture of the sheaths is consistent with the unique mode of growth, development and differentiation in
a small group of modern species among the heterocystous cyanobacteria assigned to the genus Scytonema of the section Myochrotes.
Decastronema kotori is interpreted as the fossil remains of a large filamentous microorganism, which produced and dwelt in tubular sheaths made of extracellular polymeric substances (EPS, polysaccharides with some polypeptides). The architecture of the sheaths is consistent with the unique mode of growth, development and differentiation in
a small group of modern species among the heterocystous cyanobacteria assigned to the genus Scytonema of the section Myochrotes.
![]() A comparison of the fossil with the modern representatives of that genus reveals that both employ a unique and complex mechanism to construct the upwardly divergent sheath of extracellular polymers and false branches. That mechanism involves cellular polarity, differentiation among cells to perform specialized functions and the localization of zones of active growth (meristems). Intercalary N-fixing heterocysts anchor the trichomes in the sheaths, so that their further growth produces false branches. Several fossil and modern taxa of similar morphology
exist. Their representative populations range widely in size
(Fig. 8
A comparison of the fossil with the modern representatives of that genus reveals that both employ a unique and complex mechanism to construct the upwardly divergent sheath of extracellular polymers and false branches. That mechanism involves cellular polarity, differentiation among cells to perform specialized functions and the localization of zones of active growth (meristems). Intercalary N-fixing heterocysts anchor the trichomes in the sheaths, so that their further growth produces false branches. Several fossil and modern taxa of similar morphology
exist. Their representative populations range widely in size
(Fig. 8 ![]() ).
).
![]() D. kotori is common in Santonian and lower Campanian sediments, often extraordinary abundant, usually in association with Thaumatoporella parvovesiculifera , less commonly with small foraminifera. It has also been reported in rocks
ranging in age from Aptian to Paleocene (,
1975) and found widely distributed in the Apennines (,
1975), Dinarides (Adriatic Islands, Dalmatian coast, the external Dinaridic domains; ,
1959; & ,
1990) and Helenides (, 1980).
D. kotori is common in Santonian and lower Campanian sediments, often extraordinary abundant, usually in association with Thaumatoporella parvovesiculifera , less commonly with small foraminifera. It has also been reported in rocks
ranging in age from Aptian to Paleocene (,
1975) and found widely distributed in the Apennines (,
1975), Dinarides (Adriatic Islands, Dalmatian coast, the external Dinaridic domains; ,
1959; & ,
1990) and Helenides (, 1980).
![]() Comment: The transfer of Aeolisaccus kotori to the new genus Decastronema is based on a correction of the attribution of this particular fossil. The legitimacy of the genus Aeolisaccus and its type species A. dunningtoni (,
1958) is not questioned here. But other species of that genus have been reclassified as foraminifera, e.g. A. tintinniformis 1971. A. ampliformis 1972, has been assigned tentatively to genus Erlandia, and the systematic position of A. inconstans (,
1967) and A. gracilis 1972, remains uncertain (see ,
1976). We consider the properties of Decastronema kotori unique and defined well enough to merit recognition as a discrete genus regardless of the taxonomic treatment of other species of Aeolisaccus. That
Decastronema is related to the modern Scytonema is very probable. However, because of objective limitations imposed by fossilization on the criteria for taxonomic identification, we do not recommend that the names of extant biotaxa be used for fossils.
Comment: The transfer of Aeolisaccus kotori to the new genus Decastronema is based on a correction of the attribution of this particular fossil. The legitimacy of the genus Aeolisaccus and its type species A. dunningtoni (,
1958) is not questioned here. But other species of that genus have been reclassified as foraminifera, e.g. A. tintinniformis 1971. A. ampliformis 1972, has been assigned tentatively to genus Erlandia, and the systematic position of A. inconstans (,
1967) and A. gracilis 1972, remains uncertain (see ,
1976). We consider the properties of Decastronema kotori unique and defined well enough to merit recognition as a discrete genus regardless of the taxonomic treatment of other species of Aeolisaccus. That
Decastronema is related to the modern Scytonema is very probable. However, because of objective limitations imposed by fossilization on the criteria for taxonomic identification, we do not recommend that the names of extant biotaxa be used for fossils.
![]() The proposed revision does include those species of Aeolisaccus that have been identified as cyanobacteria. In addition to Decastronema kotori, this involves only one species: Aeolisaccus barattoloi
1989, which is reassigned here as:
The proposed revision does include those species of Aeolisaccus that have been identified as cyanobacteria. In addition to Decastronema kotori, this involves only one species: Aeolisaccus barattoloi
1989, which is reassigned here as:
![]() This fossil shares most morphological characters with D. kotori. It too consists of hollow tubular segments, but is smaller and has thinner walls. The outer diameter is given as less than 33 µm. It ranges from the Maastrichtian, where it is most abundant, to the Danian, across the K/T boundary (,
1989; , 1998).
This fossil shares most morphological characters with D. kotori. It too consists of hollow tubular segments, but is smaller and has thinner walls. The outer diameter is given as less than 33 µm. It ranges from the Maastrichtian, where it is most abundant, to the Danian, across the K/T boundary (,
1989; , 1998).
![]() Comment: The existence of discrete populations of Decastronema with a broad spectrum of sizes is consistent with data on Cretaceous material from various sites and with the speciation of similar forms in comparable modern environments
(Fig. 8
Comment: The existence of discrete populations of Decastronema with a broad spectrum of sizes is consistent with data on Cretaceous material from various sites and with the speciation of similar forms in comparable modern environments
(Fig. 8 ![]() ).
).
![]() The fossil Decastronema kotori is a mineral replication of the exopolymer product of an ancient cyanobacterium. This fact raises a question regarding the nature of the process and the timing of sheath calcification and replacement.
The fossil Decastronema kotori is a mineral replication of the exopolymer product of an ancient cyanobacterium. This fact raises a question regarding the nature of the process and the timing of sheath calcification and replacement.
![]() An impressive fossil record shows that the potential for preservation of uncalcified cyanobacterial sheaths and envelopes is greater then that of the cells that produced them. The prokaryotic cells, enclosed during lifetime by an osmotically responsive plasma membrane, confined by cross-linked peptidoglycan walls, tend to collapse, shrivel and deform after death, while their sheaths contract in an orderly manner without change in shape as they dehydrate (,
1980). Thus, Proterozoic cyanobacteria, embedded and preserved in silica, consist mainly of extracellular envelopes and sheaths ( &
, 1979; & ,
1992; & ,
1998; & ,
1999), that sometimes retain pigmentation that may have screened UV-rays ( &
, 1976). This pigmentation may have been a significant protective agent during Proterozoic and Archaean times ( &
, 1991; ,
1998). However, when embedded in a carbonate, Proterozoic microbial fossils are rarely preserved in as much detail (see &
1998) and suffer more commonly from diagenetic recrystallization that obliterates the original structure (,
1976).
An impressive fossil record shows that the potential for preservation of uncalcified cyanobacterial sheaths and envelopes is greater then that of the cells that produced them. The prokaryotic cells, enclosed during lifetime by an osmotically responsive plasma membrane, confined by cross-linked peptidoglycan walls, tend to collapse, shrivel and deform after death, while their sheaths contract in an orderly manner without change in shape as they dehydrate (,
1980). Thus, Proterozoic cyanobacteria, embedded and preserved in silica, consist mainly of extracellular envelopes and sheaths ( &
, 1979; & ,
1992; & ,
1998; & ,
1999), that sometimes retain pigmentation that may have screened UV-rays ( &
, 1976). This pigmentation may have been a significant protective agent during Proterozoic and Archaean times ( &
, 1991; ,
1998). However, when embedded in a carbonate, Proterozoic microbial fossils are rarely preserved in as much detail (see &
1998) and suffer more commonly from diagenetic recrystallization that obliterates the original structure (,
1976).
![]() In contrast, most Phanerozoic fossils exist as skeletal body parts preserved in carbonates. Well preserved non-mineralized cyanobacterial fossils embedded in silica are exceptionally rare (e.g. et alii,
1997; et alii,
2002; , 2002). Therefore, it is probable that the overall change in the fossil record from Proterozoic to Phanerozoic was to some extent influenced by a concurrent change in the predominant sedimentary environment.
In contrast, most Phanerozoic fossils exist as skeletal body parts preserved in carbonates. Well preserved non-mineralized cyanobacterial fossils embedded in silica are exceptionally rare (e.g. et alii,
1997; et alii,
2002; , 2002). Therefore, it is probable that the overall change in the fossil record from Proterozoic to Phanerozoic was to some extent influenced by a concurrent change in the predominant sedimentary environment.
![]() Protists, animals and calcareous algae produce elaborate skeletal morphologies through enzymatically controlled intracellular or intercellular processes. Such precise cell-control systems are unknown in prokaryotes. Nevertheless, calcification can be promoted or inhibited by prokaryotic metabolic activities and products ( &
, 1992; & ,
1995). These biogenic influences can modify the mineralogy, size and arrangements of the precipitates, resulting in species-specific patterns ( &
, 1981; ,
1982; , 1991; &
, 1998). Among the possible mechanisms for the preservation of Decastronema by calcification are the following:
Protists, animals and calcareous algae produce elaborate skeletal morphologies through enzymatically controlled intracellular or intercellular processes. Such precise cell-control systems are unknown in prokaryotes. Nevertheless, calcification can be promoted or inhibited by prokaryotic metabolic activities and products ( &
, 1992; & ,
1995). These biogenic influences can modify the mineralogy, size and arrangements of the precipitates, resulting in species-specific patterns ( &
, 1981; ,
1982; , 1991; &
, 1998). Among the possible mechanisms for the preservation of Decastronema by calcification are the following:
![]() We conclude
that in Decastronema, templating on the polysaccharide matrix of sheaths is the mechanism most likely to have determined the sites of crystal nucleation, for the patterns of calcification
correlate closely with the sheath architecture. We suggest that calcification was a part of post-burial diagenetic
mineralization ( et alii, 2000; ,
2001). In view of the known durability of cyanobacterial sheaths, it is conceivable that the pattern of grains may have persisted through more than one diagenetic recrystallization. This supposition is supported indirectly by the fact that a subset of fossil Decastronema from the same site showed permineralization with iron rather than by calcium enrichment
(Figs. 4D-F
We conclude
that in Decastronema, templating on the polysaccharide matrix of sheaths is the mechanism most likely to have determined the sites of crystal nucleation, for the patterns of calcification
correlate closely with the sheath architecture. We suggest that calcification was a part of post-burial diagenetic
mineralization ( et alii, 2000; ,
2001). In view of the known durability of cyanobacterial sheaths, it is conceivable that the pattern of grains may have persisted through more than one diagenetic recrystallization. This supposition is supported indirectly by the fact that a subset of fossil Decastronema from the same site showed permineralization with iron rather than by calcium enrichment
(Figs. 4D-F ![]() ).
).
![]() The above interpretation does not preclude primary calcification that for Cretaceous microfossils may have been calcitic, because the ocean chemistry of the time was different from that of the modern ocean and calcification then favored calcite over aragonite ( &
, 1998). Primary calcite incorporated into the sheaths of
Decastronema may have provided some initial stability to the organo-mineral relationship that was modified subsequently.
The above interpretation does not preclude primary calcification that for Cretaceous microfossils may have been calcitic, because the ocean chemistry of the time was different from that of the modern ocean and calcification then favored calcite over aragonite ( &
, 1998). Primary calcite incorporated into the sheaths of
Decastronema may have provided some initial stability to the organo-mineral relationship that was modified subsequently.
![]() (1994) noticed a positive correlation throughout the Phanerozoic between increases in the occurrence of calcified cyanobacteria and rises in abiotic carbonate precipitates. He proposed a model that distinguishes episodes of enhanced vs. reduced cyanobacterial calcification. The occurrences of Decastronema do not appear to conform to that model's prediction for these calcified filaments were most numerous during late Cretaceous times, a period during which the rate of calcification in cyanobacteria is said to have decreased. At that time massive skeletal calcification in plankton (coccolithoforids and foraminifera; ,
2003) and benthos (e.g. rudists), may have competitively lowered environmental carbonate saturation levels. However, such changes in seawater chemistry would have mattered less if the calcification of Decastronema occurred post-depositionally.
(1994) noticed a positive correlation throughout the Phanerozoic between increases in the occurrence of calcified cyanobacteria and rises in abiotic carbonate precipitates. He proposed a model that distinguishes episodes of enhanced vs. reduced cyanobacterial calcification. The occurrences of Decastronema do not appear to conform to that model's prediction for these calcified filaments were most numerous during late Cretaceous times, a period during which the rate of calcification in cyanobacteria is said to have decreased. At that time massive skeletal calcification in plankton (coccolithoforids and foraminifera; ,
2003) and benthos (e.g. rudists), may have competitively lowered environmental carbonate saturation levels. However, such changes in seawater chemistry would have mattered less if the calcification of Decastronema occurred post-depositionally.
![]() The known occurrences of Decastronema are randomly oriented filaments and filament fragments in shallow marine
carbonates associated with marine calcareous algae and foraminifera, and commonly with the dasiclad Thaumatoporella ( &
, 1996). The fossils are in fine-grained limestone, its grains ranging from
micrite to microsparite. This lithology and association suggests that the sedimentary environment was entirely marine, but very shallow
and at extremely low energy levels
( 1975). The variablility in abundance and the random orientation of the Decastronema fragments suggest that the fossils were transported prior to deposition, and so are very probably an allochthonous element in the thanatocoenosis in which they are usually found. In addition, the fossil was never seen in growth position. Even when densely packed, the broken filaments were deposited as randomly oriented clasts. The Mirdita population, for example, accumulated in lens-shaped depressions near the contact between overlying back reef lagoonal carbonates and paleokarstic bauxite.
The known occurrences of Decastronema are randomly oriented filaments and filament fragments in shallow marine
carbonates associated with marine calcareous algae and foraminifera, and commonly with the dasiclad Thaumatoporella ( &
, 1996). The fossils are in fine-grained limestone, its grains ranging from
micrite to microsparite. This lithology and association suggests that the sedimentary environment was entirely marine, but very shallow
and at extremely low energy levels
( 1975). The variablility in abundance and the random orientation of the Decastronema fragments suggest that the fossils were transported prior to deposition, and so are very probably an allochthonous element in the thanatocoenosis in which they are usually found. In addition, the fossil was never seen in growth position. Even when densely packed, the broken filaments were deposited as randomly oriented clasts. The Mirdita population, for example, accumulated in lens-shaped depressions near the contact between overlying back reef lagoonal carbonates and paleokarstic bauxite.
![]() The comparison with modern Scytonema supports a subaerial habitat for the fossil. Today, thick-layered sheaths are characteristic of subaerial species of Scytonema, whereas the sheaths of submersed species are thin (,
1934). So the habitat of Decastronema with its thick and divergent sheaths was probably subaerial.
The comparison with modern Scytonema supports a subaerial habitat for the fossil. Today, thick-layered sheaths are characteristic of subaerial species of Scytonema, whereas the sheaths of submersed species are thin (,
1934). So the habitat of Decastronema with its thick and divergent sheaths was probably subaerial.
![]() Modern Scytonema, considered a likely counterpart of Decastronema, grows on intertidal flats. It forms extensive mats on the west coast of Andros Island, Bahamas, covering many square km around interspersed bushes of Rhizophora mangle
(Fig. 9
Modern Scytonema, considered a likely counterpart of Decastronema, grows on intertidal flats. It forms extensive mats on the west coast of Andros Island, Bahamas, covering many square km around interspersed bushes of Rhizophora mangle
(Fig. 9 ![]() ). Periodic storms often disrupt and displace these intertidal Scytonema mats and bury them in layers of fine-grade carbonate mud. Short cores taken from the mud flats
(Fig. 9, inset
). Periodic storms often disrupt and displace these intertidal Scytonema mats and bury them in layers of fine-grade carbonate mud. Short cores taken from the mud flats
(Fig. 9, inset ![]() ) show dark layers of dislocated and randomly oriented Scytonema filaments between layers of carbonate mud with a few complete colonies preserved (R.N. , personal communication). However, disrupted and fragmented Scytonema filaments are also exported by tides and distributed over a much larger area where these fragments are buried in a shallow subtidal setting
(Fig. 10
) show dark layers of dislocated and randomly oriented Scytonema filaments between layers of carbonate mud with a few complete colonies preserved (R.N. , personal communication). However, disrupted and fragmented Scytonema filaments are also exported by tides and distributed over a much larger area where these fragments are buried in a shallow subtidal setting
(Fig. 10 ![]() ). So we assume that the Cretaceous Decastronema lived in an intertidal habitat, similar to that on the west coast of the Andros Island
(Fig. 11
). So we assume that the Cretaceous Decastronema lived in an intertidal habitat, similar to that on the west coast of the Andros Island
(Fig. 11 ![]() ) but after death was transported to adjacent shallow areas of the carbonate platform where it was buried and fossilized.
) but after death was transported to adjacent shallow areas of the carbonate platform where it was buried and fossilized.
![]() Here we have compared cyanobacteria of similar structure and comparable habitat separated by 60-70 million years of geological time. Andros Island is the largest of a number of emergent portions of the Bahama carbonate platform, surrounded by extensive areas of shallow waters not more than a few meters deep. The sediment build-up on the platform, continuous since Mesozoic times, shows that its constructon kept abreast
of repeated changes in sea level. In southern Europe where Decastronema
lived the development of carbonate platforms of similar age and lithology was
interrupted by Alpine orogeny following the collision of African and Eurasian
continental plates. The carbonate environments compared here may have had a common origin as parts of a series of Mesozoic platforms along the southern margins of the Tethys Sea (,
1970). The western portion of these platforms escaped destruction by Alpine orogenesis as the Atlantic Ocean widened, and continued to exist on the stable trailing end of the North American continent. Contemporaneous strata on both platforms were analyzed and found to be lithologically identical ( et alii,
1975). Here we add paleontological support to the similarities in lithological and paleoenvironmental properties reported independently by others.
Here we have compared cyanobacteria of similar structure and comparable habitat separated by 60-70 million years of geological time. Andros Island is the largest of a number of emergent portions of the Bahama carbonate platform, surrounded by extensive areas of shallow waters not more than a few meters deep. The sediment build-up on the platform, continuous since Mesozoic times, shows that its constructon kept abreast
of repeated changes in sea level. In southern Europe where Decastronema
lived the development of carbonate platforms of similar age and lithology was
interrupted by Alpine orogeny following the collision of African and Eurasian
continental plates. The carbonate environments compared here may have had a common origin as parts of a series of Mesozoic platforms along the southern margins of the Tethys Sea (,
1970). The western portion of these platforms escaped destruction by Alpine orogenesis as the Atlantic Ocean widened, and continued to exist on the stable trailing end of the North American continent. Contemporaneous strata on both platforms were analyzed and found to be lithologically identical ( et alii,
1975). Here we add paleontological support to the similarities in lithological and paleoenvironmental properties reported independently by others.
![]() Decastronema kotori was found first in the Turonian rocks (Upper Cretaceous) of the external Dinarides (,
1959). Subsequently it was found in strata ranging from uppermost Aptian to Paleocene, with a maximum abundance in the Senonian (reviewed by ,
1975). Although the organism survived the massive extinctions at the Cretaceous-Tertiary transition, it has not yet been encountered in earlier or later deposits. This is unusual for a member of cyanobacteria, which as a group diversified early in the Proterozoic and persisted over time while maintaining their conservative phenotypic properties. Heterocystous cyanobacteria, of which Decastronema is one, were reported from Devonian Rhynie chert (
& , 1921), but may have evolved during the Mesoproterozoic, between 1300 and 1500 million years ago, as suggested by the findings of the fossil Archaeoellipsoides, interpreted as akinetes of heterocystous cyanobacteria similar to
Anabaena ( et alii, 1995).
Decastronema kotori was found first in the Turonian rocks (Upper Cretaceous) of the external Dinarides (,
1959). Subsequently it was found in strata ranging from uppermost Aptian to Paleocene, with a maximum abundance in the Senonian (reviewed by ,
1975). Although the organism survived the massive extinctions at the Cretaceous-Tertiary transition, it has not yet been encountered in earlier or later deposits. This is unusual for a member of cyanobacteria, which as a group diversified early in the Proterozoic and persisted over time while maintaining their conservative phenotypic properties. Heterocystous cyanobacteria, of which Decastronema is one, were reported from Devonian Rhynie chert (
& , 1921), but may have evolved during the Mesoproterozoic, between 1300 and 1500 million years ago, as suggested by the findings of the fossil Archaeoellipsoides, interpreted as akinetes of heterocystous cyanobacteria similar to
Anabaena ( et alii, 1995).
![]() The peculiar divergent layering of the sheath of Scytonema and Decastronema along with the false branching of filaments is a stage in the evolution of filamentous cyanobacteria during which the cells differentiate to assume different forms and functions: The
'vegetative' trichome cells fix carbon and grow in localized meristematic zones, the heterocysts fix nitrogen and the akinetes store nutrients and act as resting spores. These properties in modern cyanobacteria serve as phylogenetic markers. The interpretation of the architecture of the Cretaceous fossil is consistent with the presence of these elements in modern heterocystous cyanobacteria.
The peculiar divergent layering of the sheath of Scytonema and Decastronema along with the false branching of filaments is a stage in the evolution of filamentous cyanobacteria during which the cells differentiate to assume different forms and functions: The
'vegetative' trichome cells fix carbon and grow in localized meristematic zones, the heterocysts fix nitrogen and the akinetes store nutrients and act as resting spores. These properties in modern cyanobacteria serve as phylogenetic markers. The interpretation of the architecture of the Cretaceous fossil is consistent with the presence of these elements in modern heterocystous cyanobacteria.
![]() The time constraints
on the range of Decastronema may be environmental as well as evolutionary. If our conjectures based on comparisons with modern Scytonema are correct, the fossil lived on the intertidal flats adjacent to land. The intertidal areas are normally very narrow strips of the coast. Consequently it is possible that the fossil has been overlooked in stratigraphic surveys due to its narrowly defined ecological niche. Environments within the intertidal range are especially vulnerable to frequent changes in sea level that limit recording of the occurrence of intertidal biota to episodes that geologically are very short. Short-term sea level oscillations that follow
cycles, as recorded in microstratigraphic analyses ( et alii,
1994;
et alii, 1992,
1997; et alii,
2002), may have caused many repeated short exposures and submersions on carbonate platforms as well as significant lateral migration of habitats and their biota. Each such change, although it has an ecologically dramatic impact, is no more than an instant on the geological time scale.
The time constraints
on the range of Decastronema may be environmental as well as evolutionary. If our conjectures based on comparisons with modern Scytonema are correct, the fossil lived on the intertidal flats adjacent to land. The intertidal areas are normally very narrow strips of the coast. Consequently it is possible that the fossil has been overlooked in stratigraphic surveys due to its narrowly defined ecological niche. Environments within the intertidal range are especially vulnerable to frequent changes in sea level that limit recording of the occurrence of intertidal biota to episodes that geologically are very short. Short-term sea level oscillations that follow
cycles, as recorded in microstratigraphic analyses ( et alii,
1994;
et alii, 1992,
1997; et alii,
2002), may have caused many repeated short exposures and submersions on carbonate platforms as well as significant lateral migration of habitats and their biota. Each such change, although it has an ecologically dramatic impact, is no more than an instant on the geological time scale.
![]() The long-term survival of shallow water biota on carbonate platforms requires that sedimentation be successful in matching subsidence. Their demise may be caused by drowning, as was the case with mid-oceanic platforms ( et alii,
1998), or by major tectonic changes associated with continent collisions, as was the case with the Periadriatic carbonate platforms. Carbonate platforms such as the Bahamas that have existed for a long time are the exception. Their longevity is due to the relative stability of conditions on the trailing side of the continent.
The long-term survival of shallow water biota on carbonate platforms requires that sedimentation be successful in matching subsidence. Their demise may be caused by drowning, as was the case with mid-oceanic platforms ( et alii,
1998), or by major tectonic changes associated with continent collisions, as was the case with the Periadriatic carbonate platforms. Carbonate platforms such as the Bahamas that have existed for a long time are the exception. Their longevity is due to the relative stability of conditions on the trailing side of the continent.
![]() Microorganisms on carbonate platforms have been subjected to significant long and short-term changes in environment. Prokaryotes such as cyanobacteria have responded by adaptations to environmental instabilities and have re-occupied their niches after catastrophic changes, including periods of major extinctions. Their record as fossils continues unbroken although it is accompanied by changes in the composition of successful but short-lived narrowly specialized eukaryotic microbiota,
e.g. coccolithophorids, foraminifera and calcareous algae. We expect, therefore, that Decastronema-type fossils will eventually be found in peritidal settings throughout the Phanerozoic and perhaps earlier.
Microorganisms on carbonate platforms have been subjected to significant long and short-term changes in environment. Prokaryotes such as cyanobacteria have responded by adaptations to environmental instabilities and have re-occupied their niches after catastrophic changes, including periods of major extinctions. Their record as fossils continues unbroken although it is accompanied by changes in the composition of successful but short-lived narrowly specialized eukaryotic microbiota,
e.g. coccolithophorids, foraminifera and calcareous algae. We expect, therefore, that Decastronema-type fossils will eventually be found in peritidal settings throughout the Phanerozoic and perhaps earlier.
![]() The authors are thankful for the materials and data used in comparative aspect of this work, and especially for many stimulating discussions with Piero , Bruno
, Lucia , Andrew and Gilbert as well as anonymous reviewers are thanked for constructive criticism of the manuscript. Special thanks
are due Nestor for language corrections and significant improvement of the text. The international and interdisciplinary collaboration was supported by Hanse Institute for Advanced Study, Alexander von Foundation, and National Invited Professors Program of the University of Marseille.
The authors are thankful for the materials and data used in comparative aspect of this work, and especially for many stimulating discussions with Piero , Bruno
, Lucia , Andrew and Gilbert as well as anonymous reviewers are thanked for constructive criticism of the manuscript. Special thanks
are due Nestor for language corrections and significant improvement of the text. The international and interdisciplinary collaboration was supported by Hanse Institute for Advanced Study, Alexander von Foundation, and National Invited Professors Program of the University of Marseille.
G., A. & J. (1999).- Calcification in cyanobacterial biofilms of alkaline salt lakes.- European Journal of Phycology, Oxford, vol. 34, n° 4, pp. 393-403.
F. (1998).- Dasycladacean green algae and microproblematica of the uppermost Cretaceous-Paleocene in the Karst area (NE Italy and Slovenia).- Dela - Opera SAZU (Slovenska Akademija Znanosti in Umetnosti), Ljubljana, opus 4, 34/2, pp. 65-127.
F. & A. (1996).- Dasycladaleans and depositional environments of the Upper Triassic-Liassic carbonate platform of the Gran Sasso (Central Apennines, Italy).- Facies, Erlangen, 35, pp. 163-208.
Y. (1934).- False branching and sheath-structure in the Myxophyceae, with special reference to the Scytonemataceae.- Archiv für Prostistenkunde, Jena, vol. 81 (1933-1934), pp. 243-283.
M. (1933).- The algal sediments of Andros Island, Bahamas.- Philosophical Transactions of the Royal Society of London, (series B), vol. 222, pp. 165-192.
J.B.E. & C.G.M. (1887).- Révision des Nostocacées hétérocystées.- Annales des Sciences Naturelles, Botanique, Paris, (sér. 7), 5, p. 51-129.
O., G., C. & E.P. (2003).- Bacterially induced mineralization of calcium carbonate in terrestrial environments: The role of exopolysaccharides and amino acids.- Journal of Sedimentary Research, Boulder, vol. 73, n° 2, pp. 485-490.
D.E.G. (2003).- The role of decay and mineralization in the preservation of soft-bodied fossils.- Annual Review of Earth and Planetary Sciences, Palo Alto, vol. 31, pp. 275-301.
F., M., A., B., V., R. & G. (2002).- Cyclostratigraphy and high-frequency carbon isotope fluctuations in Upper Cretaceous shallow-water carbonates, southern Italy.- Sedimentology, Oxford, vol. 49, n° 6, pp. 1321-1337.
G.F., A., D.D., P. & P. (1998).- Development and demise of mid-oceanic carbonate platforms, Wodejebato Guyot (NW Pacific).- In: G.F. & P.J. (eds.), Reefs and carbonate platforms in the Pacific and Indian oceans.- International Association of Sedimentologists, Special Publication, Oxford, 25, pp. 39-67.
E., H., S.R.S., A.H. & S. (2002).- 70 Ma nonmarine diatoms from northern Mexico.- Geology, Boulder, vol. 30, n° 3, pp. 279-281.
H.S. & C. (1992).- Bacterially induced lithification of microbial mats.- Palaios, Tulsa, vol. 7, n° 3, pp. 277-293.
A. (1982).- Ultrastructure d'une cyanophycée aérienne calcifiée cavernicole: Geitleria calcarea .- Hydrobiologia, Amsterdam, vol. 97, n° 3, pp. 255-274.
B. (1970).- Evoluzione geotettonica comparata tra alcune piattaforme carbonatiche dei Mediterranei Europeo ed Americano.- Atti del'Accademia Pontaniana, Nuova Serie, Napoli, vol. 20, pp. 1-34.
B., P., C. & L. (1975).- Bahamian and Apenninic limestones of identical lithofacies and age.- American Association of Petroleum Geology, Bulletin, Tulsa, vol. 59, n° 3, p. 524-533.
B., V. & R. (1992).- A cm-scale study of shallow water cretaceous deposits formed under high frequency eustatic regime. Monti di Sarno (southern Italy). A sedimentologic approach to microstratigraphy.- Bolletino della Società Geologica Italiana, Roma, vol. 111, pp. 399-407.
B., V., S. & N. (1997).- Hierarchy of high-frequency orbital cycles in Cretaceous carbonate platform strata.- Sedimentary Geology, Amsterdam, vol. 113, pp. 169-193.
S.M., C., S.W., A.M., M.J. & E.N. (1999).- Directed nucleation of calcite at a crystal-imprinted polymer surface.- Nature, London, vol. 398, n° 6725, pp. 312-316.
P. (1975).- Osservazioni su Aeolisaccus kotori 1959 (Cyanoschizophyta).- Bolletino della Società dei Naturalisti in Napoli, vol. LXXXIV, pp. 1-23.
P. (1989).- On some foraminifera and algae in Apennine Upper Cretaceous and Paleocene.- Memorie della Società Geologica Italiana, Roma, vol. 40 (1987), pp. 109-124.
C. & J. (1995).- From biominerals to organominerals: the example of the modern lacustrine calcareous stromatolites from Polynesian atolls.- Bulletin de l'Institut océanographique, Monaco, n° spéc. 14, pp. 265-271.
C., P.T., L.K. & R.P. (2004).- Microbe-mineral interactions: early carbonate precipitation in a hypersaline lake (Eleuthera Island, Bahamas).- Sedimentology, Oxford, vol. 51, n° 4, pp. 745-765.
G.F. (1958).- Fossil microproblematica from the Middle East.- Micropaleontology, New York, vol. 4, n° 4, pp. 419-428.
J.J. (1980).- Les zones de Gavrovo–Tripolitza et du Pinde–Olonos (Grèce continental et Péloponnèse du Nord). Évolution d'une plate-forme et d'un bassin dans leur cadre alpin.- Société géologique du Nord, Publication, Lille, n° 4, 651 p.
P. & E.P. (1998).- Freshwater organisms that build stromatolites: a synopsis of biocrystallization by prokaryotic and eukaryotic algae.- Sedimentology, Oxford, vol. 45, n° 3, pp. 535-563.
F. (1998).- Solar ultraviolet and the evolutionary history of cyanobacteria.- Origins of Life and Evolution of the Biosphere, Amsterdam, vol. 28, n° 3, pp. 321-347.
F. & R.W. (1991).- Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment.- Journal of Phycology, vol. 27, pp. 395-409
P., G., S. & S. (2004).- Biochemical control of calcium carbonate precipitation in modern lagoonal microbialites, Tikehau Atoll, French Polynesia.- Journal of Sedimentary Research, Tulsa, vol. 74, n° 4, pp. 462-478.
S. (1980).- Early photosynthetic microorganisms and environmental evolution.- In: R. (ed.), Life science and space research, COSPAR 18.- Pergamon Press, Oxford, pp. 101-107.
S. & E.S. (1976).- Interpretation of microbial fossils with special reference to the Precambrian.- In: E. (ed.), Fossil algae.- Springer-Verlag, Heidelberg, pp. 1-14.
S. & S.E. (1981).- Biogenically formed aragonite concretions in marine Rivularia.- In: C.L.V. (ed.), Phanerozoic stromatolites.- Springer-Verlag, Heidelberg, pp. 209-229.
S., M. & L. (1996).- Developmental aspects of branching in filamentous Cyanophyta/Cyanobacteria.- Algological Studies, Stuttgart, vol. 83, pp. 303-329.
S. & H.J. (1976).- Comparison of Holocene and mid-Precambrian Entophysalidaceae (Cyanophyta) in stromatolitic algal mats; cell division and degradation.- Journal of Paleontology, Tulsa, vol. 50, n° 6, pp. 1074-1082.
S. & L. (1999).- Early Cyanobacterial fossil record: preservation, palaeoenvironments and identification.- European Journal of Phycology, Cambridge, vol. 34, n° 4, pp. 339-348.
S., L. & K.M. (2000).- Cyanobacteria: Architects of sedimentary structures.- In: R.E. & S.M. (eds.), Microbial sediments.- Springer-Verlag, Heidelberg, pp. 57-67.
S., V.N. & A.H. (1995).- Mesoproterozoic Archaeoellipsoides: akinetes of heterocystous cyanobacteria.- Lethaia, Oslo, vol. 28, pp. 285-298.
I. & V. (1990).- Upper Cretaceous stratigraphy the Island of Brač within the geodynamic evolution of the Adriatic carbonate platform.- Djela Jugoslavenske Akademije Znanosti i Umjetnosti, Razred prirodnih znanosti, Zagreb, Knj. 69, pp. 1-60.
H.J. (1976).- Precambrian microflora, Belcher Island, Canada; significance and systematics.- Journal of Paleontology, Tulsa, vol. 50, n° 6, pp. 1040–1073.
H. (2002).- The Rhynie Chert - The oldest and most completely preserved terrestrial ecosystem.- In: U. & W.D. (eds.), Secrets of petrified plants.- D'Oro Verlag, Heppenheim, pp. 22-27.
R. & W.H. (1921).- On Old Red Sandstone plants showing structure, from the Rhynie Chert bed, Aberdeenshire. Part V. The Thallophyta.- Transactions of the Royal Society of Edinburgh, vol. 52(33), pp. 855-902.
A.H. (2003).- Biomineralization and evolutionary history.- Reviews in Mineralogy and Geochemistry, Chantilly, vol. 54, pp. 329-356.
A.H. & S. (1979).- Anatomy and taphonomy of a Precambrian algal stromatolite.- Precambrian Research, Amsterdam, vol. 10, n° 1-2, pp. 115-151.
A.H. & S. (1992).- Modern and ancient cyanobacteria. In: M., S., M.M., D.M. & P.A. (eds.), Early organic evolution: Implications for mineral and energy resources.- Springer-Verlag, Heidelberg, pp. 450-462.
A.H., S., J. & K. (1986).- Organically preserved microbial endoliths from the late Proterozoic of East Greenland.- Nature, London, vol. 321, n° 6073, pp. 856-857.
A.H. & M.A. (1998).- The genesis and time distribution of two distinctive Proterozoic stromatolite microstructures.- Palaios, Tulsa, vol. 13, n° 5, pp. 408-422.
G., B., V. & M. (1994).- Fourier evidence for high-frequency astronomical cycles recorded in Early Cretaceous carbonate platform strata, Monte Maggiore, southern Apennines, Italy.- International Association of Sedimentologists, Special Publication, Oxford, 19, pp. 77-85.
M.U.E. (1992).- The biology of carbonate precipitation by cyanobacteria.- Facies, Erlangen, 26, pp. 81–102.
M. (2000).- Calcification in cyanobacteria. In: R.E. & S.M. (eds.), Microbial sediments.- Springer-Verlag, Heidelberg, pp. 50-56.
C.L.V. (1967).- Distribution and structure of recent stromatolitic algal mats, eastern Andros Island, Bahamas.- Annales de Société Géologique de Belgique, Liège, t. 90, pp. 55-99.
J.W. & Q. (1996).- Factors influencing the grain size distribution of authigenic minerals.- American Journal of Science, New Haven, vol. 296, n° 9, pp. 989-1003.
J. (1991).- Biologie und Ökologie von drei rezenter süsswasser-Rivularien (Cyanobakterien) – Übertragbarkeit artspezifischer Verkalkungsstrukturen auf fossile Formen.- Göttinger Arbeiten zur Geologie und Paläontologie, Band 50, 86 p.
H.W., T.F. & R.P. (2001).- Bacterially mediated precipitation in marine stromatolites.- Environmental Microbiology, vol. 3, n° 2, pp. 123-130.
A. (1991).- Calcification processes in algae and cyanobacteria.- In: R. (ed.), Calcareous algae and stromatolites.- Springer-Verlag, Heidelberg, pp. 3-20.
B.R. (2001).- Calcification of cyanobacterial filaments: Girvanella and the origin of lower Paleozoic lime mud.- Geology, Boulder, vol. 29, n° 9, pp. 763-766.
R. (1959).- Nekoliko problematičnih mikrofosila iz dinarske Krede (Some problematic microfossils from the Dinarian Cretaceous).- Zavod za Geološka i Geofizička Istraživanja, Vesnik, Beograd, (Serija A), t. XVII, pp. 87-92.
R.R. (1967).- O problematičnim mikrofosilima iz jure i krede Dinarida (Sur les microfossiles problématiques du Jurassique et du Crétacé des Dinarides).- Zavod za Geološka i Geofizička Istraživanja, Vesnik, Beograd, (Serija A), t. XXIV/XXV, pp. 269-280.
R. (1972).- Nubekularide u Juri i Kredi Dinarida i bilješka o vrsti Aeolisaccus kotori (Nubécularides du Jurassique et du Crétacé des Dinarides et une note sur l'espèce Aeolisaccus kotori ).- Zavod za Geološka i Geofizička Istraživanja, Vesnik, Beograd, (Serija A), t. XXIX/XXX, pp. 235-261.
R. & B. (1999).- An outline of the geology of external Dinarides and their Mesozoic–Early Tertiary facies.- Rendiconti dell'Accademia delle Scienze Fisiche e Matematiche, Napoli, vol. 66, pp. 181-243.
R. (1991).- Classification of microbial carbonates. In: R. (ed.), Calcareous algae and stromatolites.- Springer-Verlag, Heidelberg, pp. 21-51.
R. (1994).- Evolution of algal and cyanobacterial calcification.- In: S. (ed.), Early life on earth.- Nobel Symposium 84, Columbia University Press, New York, pp. 426-438.
J.W. & C. (eds.) (1992).- The Proterozoic biosphere. A multidisciplinary study.- Cambridge University Press, 1374 p.
L. & S. (1998).- Multi-trichomous cyanobacterial microfossils from the Mesoproterozoic Gaoyuzhuang Formation, China: Paleoecological and taxonomic implications.- Lethaia, Oslo, vol. 31, n° 3, pp. 169-184.
S., G., S. & T. (2001).- Microbialites in a modern lagoonal environment: nature and distribution, Tikehau atoll (French Polynesia).- Palaeogeography, Palaeoclimatology, Palaeoecology, vol. 175, n° 1-4, pp. 103-124.
S.M. & L.A. (1998).- Secular oscillations in the carbonate mineralogy of reef-building and sediment-producing organisms driven by tectonically forced shifts in seawater chemistry.- Palaeogeography, Palaeoclimatology, Palaeoecology, Amsterdam, vol. 144, n° 1-2, pp. 3–19.
T.N., H. & H. (1997).- A cyanolichen from the Lower Devonian Rhynie Chert.- American Journal of Botany, St. Louis, Vol. 84, n° 7, pp. 992-1004.
J. & C. (1995).- Non-biologically supported organomineralization.- Bulletin de l'Institut océanographique, Monaco, n° spéc. 14, pp. 203-236.
E.C., N.P. & G.M. (2000).- Taphonomic control on microstructure in early Neoproterozoic reefal stromatolites and thrombolites.- Palaios, Tulsa, vol. 15, n° 2, pp. 87-111.
P.T., R.P. & B.M. (2000).- Microscale observations of sulfate reduction: Correlation of microbial activity with lithified micritic laminae in modern marine stromatolites.- Geology, Boulder, vol. 28, n° 10, pp. 919-922.
I., J., I. & D. (2002).- The Karst Dinarides are composed of relics of a single Mesozoic platform: Facts and consequences.- Geologia Croatica, Zagreb, vol. 55/2, pp. 171-183.
L. (1976).- Les Foraminifères du Trias. Essai de synthèse et corrélation entre les domaines mésogéens européen et asiatique.- Rivista Italiana di Paleontologia e Stratigrafia, Milano, vol. 82, pp. 1-258.
Click on thumbnail to enlarge the image.
Figure 1: Area of study, the frame of the map sheet Orahovac and the outlines of the countries superimposed on NASA World Wind satellite image.

Click on thumbnail to enlarge the image.
Figure 2: A simplified presentation of the geological setting of the Decastronema sampling site above the bauxite palaeokarst horizon of the Metohia, Mirdita zone, internal Dinarides. 1. Karstified Lower Cenomanian limestone: Pseudorhapydionina dubia (), Rotalia mesogeensis , Nummoloculina sp. and other miliolids; 2. Three types of Santonian or Lowermost Campanian limestones were recognized: a) limestone with numerous Fe ooids and Rotorbinella scarsellai , discorbids, and rare Clypeina dusanbrstinai ; b) limestone with dispersed fertile ampullae of Neomeris and c) Limestone with foraminifera Accordiella conica , Pseudocyclammina sphaeroidea , Dicyclina and Nezzazatinella sp.; red – bauxite; black - lentiform level, in the lower part with layers of densely packed and well sorted fragments of Decastronema kotori.
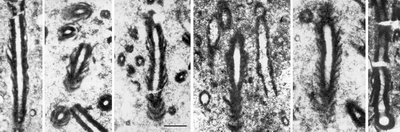
Click on thumbnail to enlarge the image.
Figure 3: Petrographic thin section of Decastronema kotori () combinatio nova: (A) A filament in longitudinal section; note the thinning and bending of diverging outer layers of the wall; (B) Fragmented filaments of varied orientation in transverse and oblique sections; note the conspicuously clear lumina; (C–E) Filaments in nearly longitudinal section, becoming tangential at the lower end where the filaments curve out of the plane of the section; (F) Filament with "terminal chamber", representing a combination of longitudinal and transversal section at the position of a false branch (modified from , 1975). Scale bar in C is 50 µm long for all pictures.

Click on thumbnail to enlarge the image.
Figure 4:
SEM images of Decastronema kotori on polished slightly etched rock surfaces: (A) View of a filament almost 1 mm long with upward diverging wall layers;
(B-C) Oblique, transverse and longitudinal sections through filaments showing a concentric, upward divergent layering of the wall; the wall is comprised of micritic grains, whereas the lumen is filled with microsparite similar to that occupying pore spaces in the surrounding sediment; (D) Two fossil filaments etched from the matrix by prolonged application of acid. The wall appears spongy for it consists of a Fe-enriched non-crystalline
material; (E-F) A similar etching of filaments in longitudinal and oblique sections; (G) Enlargement of a negative print of B, as negative, showing grain relationships in the micritically preserved sheath; (H) Detail of E, showing the spongy Fe-enriched texture of the wall; note the fine submicron-size porosity; (I) Detail of D: The upper filament, a nearly transverse section viewed from below, shows the core and two layers of the wall (comp. with
Fig. 5B ![]() ). The scale bar in A is 50 µm long; the scale bar in F is 50 µm long valid for B-F; the scale bars in G-I are 10 µm long.
). The scale bar in A is 50 µm long; the scale bar in F is 50 µm long valid for B-F; the scale bars in G-I are 10 µm long.

Click on thumbnail to enlarge the image.
Figure 5:
SEM of critical-point-dried specimens of Recent (modern) Scytonema sp. from the mats covering intertidal carbonate mud flats on the west coast of Andros Island,
Bahamas. (A) Intact, upward curving filaments; the surface shows no appreciable carbonate precipitation. (B) View down a fractured filament of Scytonema showing the concentric arrangement of layered sheath enveloping a trichome cf.
Fig. 4I ![]() . (C) Oblique view of another fractured filament with a still turgescent cell in the center of concentric sheath layers. Note the reticulate texture of the polysaccharide in the upper part of the picture. Scale bars are 10 µm long; the bar in C is valid for B.
. (C) Oblique view of another fractured filament with a still turgescent cell in the center of concentric sheath layers. Note the reticulate texture of the polysaccharide in the upper part of the picture. Scale bars are 10 µm long; the bar in C is valid for B.
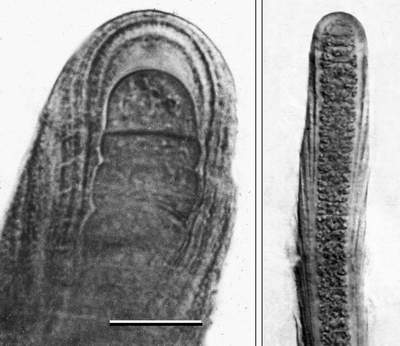
Click on thumbnail to enlarge the image.
Figure 6: Filament tips of modern Scytonema sp. under light microscope showing the development of divergent sheath collars: (A) The tip of the trichome excretes layers of EPS that first stretch and then break as the trichome extends. (B) Sheath production is most intense at the tip and less basipetally. The lower parts of the trichome are enveloped by a series of inserted cones. Note the thinning of envelope margines in both pictures. The scale bar in A is 10 µm for A and 30 µm for B.
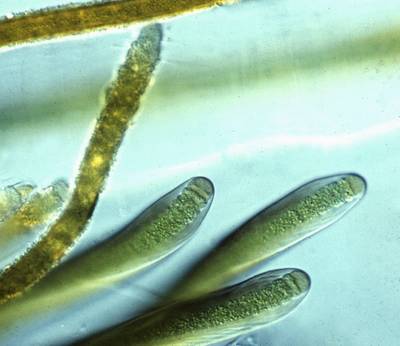
Click on thumbnail to enlarge the image.
Figure 7: Two species of Scytonema growing on cliffs wetted by freshwater. They show distinction in size, sheath construction and the ability to support carbonate precipitation. The smaller one (left) is Scytonema julianum with sheaths encrusted with calcite crystals, the larger one (right) is S. myochrous producing divergently layered non-calcifying sheaths. Scale bar is 10 µm.
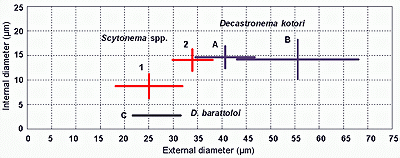
Click on thumbnail to enlarge the image.
Figure 8: Plot of the size relationships between external diameters (x) vs. internal diameters (y) of filaments in fossil populations of Decastronema (A–C) and Recent populations of Scytonema from Andros Island, Bahamas (1–3). Mean values plot at the point of intersection in each of the cross diagrams, with arms extended for one standard deviation on each side of the mean. The total range of data for each population is inside a field of two standard deviations on each side of the mean.

Click on thumbnail to enlarge the images.
Figure 9: Scytonema mats around mangrove bushes on Intertidal flats of Andros Island, Bahamas form a dense brown cover over white carbonate mud. Inset: Core taken from the same area showing dark Scytonema fragments interlayered by carbonate mud.

Click on thumbnail to enlarge the image.
Figure 10: Aerial view of tidal creeks on the NW coast of Andros Island, Bahamas, rimmed by Scytonema cover (reddish brown) around bright carbonate mud in shallow ponds.

Click on thumbnail to enlarge the image.
Figure 11: Westward view of the ponded intertidal mud flats of Andros Island, a possible analogue of the environmental setting of the Cretaceous Decastronema.