Contents
[Introduction] [Material and methods]
[Results] [Discussion] and ...
[Bibliographic references]
Department of Geology, University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
Manuscript online since March 15, 2011
Most known serpulid tube ultrastructures in contact with an organic inner tube lining do not show the direction in which they developed. But spherulitic prismatic structures found in the innermost part of the tube wall of Recent Crucigera websteri, C. zygophora, Floriprotis sabiuraensis, and Pyrgopolon ctenactis indicate that the structure grew toward the organic inner tube lining and also toward the tube's lumen. Similar directions of growth for this structure are seen in Hydroides sp. from the Miocene of Austria. Growth towards the tube's lumen is opposite to what one would expect if the organic inner tube lining is being used as a scaffold for the biomineralization of CaCO3.
Biomineralization; cuticles; Serpulidae; tube ultrastructure.
O. (2011).- The role of an internal organic tube lining in the biomineralization of serpulid tubes.- Carnets de Géologie / Notebooks on Geology, Brest, Letter 2011/01 (CG2011_L01)
Le rôle d'un revêtement organique interne dans la biominéralisation de tubes de serpulidés.- La plupart des ultrastructures biominéralisées observées au contact du revêtement organique interne du tube des serpulidés ne présentent pas d'indices de croissance directionnelle. Cependant des structures sphérolitiques prismatiques observées sur la face interne du tube de formes modernes telles que Crucigera websteri, C. zygophora, Floriprotis sabiuraensis et Pyrgopolon ctenactis indiquent que cette structuration s'accroît en direction de la couche organique interne et aussi vers la lumière du tube. Cette direction de croissance s'observe également chez un Hydroides sp. du Miocène d'Autriche. Elle est contraire à celle qu'on attendrait dans l'hypothèse où le revêtement organique interne ne jouerait que le rôle de simple support dans la biominéralisation du tube en CaCO3.
Biominéralisations ; cuticules ; Serpulidae ; ultrastructure du tube.
CaCO3-walled tubes are found in all Serpulidae, in a few of the species of the genus Glomerula of the family Sabellidae, and in a few species of the Cirratulidae. They evolved independently, appearing first in serpulids of Middle Triassic age ( & , 2009), then in sabellids from the Early Jurassic ( et alii, 2008a) and finally in cirratulids dated Oligocene (, 2000). Mineralized serpulid tubes can be aragonitic or calcitic and some are composed of both minerals ( & , 1973; et alii, 2008b), but sabellids and cirratulids build only aragonitic tubes ( et alii, 2008b; et alii, 2010). Calcareous polychaetes lack an external organic covering like the periostracum of molluscs and brachiopods, the epidermis of echinoderms, or the cuticle of bryozoans. However, serpulids possess an internal organic tube lining (, 1993; et alii, 2008b; et alii, 2010).
Biominerals in marine invertebrate skeletons (e.g. those of annelids, brachiopods, bryozoans and corals, etc.) are natural composites of inorganic and organic components. The organic components have a fundamental role in the formation, structure and emplacement of the mineral phases ( & , 2003). The insoluble organic tube lining (in EDTA: ethylenediaminetetraacetic acid) of serpulids has been identified as being composed in part of collagen-containing fibres aligned in a criss-crossed structure with carboxylated and sulphated polysaccharides ( et alii, 2010). et alii (2010) hypothesized that Hydroides dianthus could use its organic tube lining as a scaffold for the biomineralization of CaCO3. They found these collagen-containing fibres to be associated at the tube wall interface with CaCO3 crystals, and they detected elements consistent with Mg-calcite in the fibres and on the smooth sheet at the lumen interface. et alii (1985) showed that the decalcified insoluble organic matrix of H. dianthus was capable of becoming recalcified in an inorganic solution, mimicking the ion concentrations found in molluscan extrapallial fluid. However, the concept of organic tube lining as a scaffold for biomineralization is not consonant with the formation of the tube behind the backward folded collar of the serpulids (, 1958; ten & , 2009).
The several skeletal ultrastructural fabrics in serpulids when compared to those of sabellids and cirratulids raises a question as to whether or not this diversity could be related to the presence of an organic inner tube lining only in serpulids. The aim of this paper is to test the hypothesis that serpulids use their organic tube lining as a scaffold for the biomineralization of CaCO3 using the tube's ultrastructure as a foundation.
An SEM photo database of 45 recent (global) and 20 fossil serpulid tube ultrastructures (ranging in age from the Jurassic through the Miocene of Europe) was searched in order to determine the direction of crystal growth in the innermost layer of the tube wall. All samples with a mineralized ultrastructure were embedded in epoxy resin, polished in longitudinal and transversal directions, and then etched with 1% acetic acid for five to ten minutes prior to SEM examination. The lining of the organic tube was studied on untreated natural breakage surfaces.
Scanning electron microscopy was done with a Hitachi S-4300 at the Swedish Museum of Natural History, Stockholm, and the etched samples and organic lining were viewed with a Zeiss 940D microscope at the Department of Geology, University of Tartu.
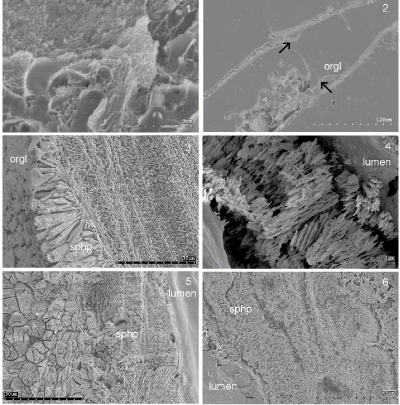
The organic tube lining was found to be in contact with nine of fourteen currently known serpulid tube ultrastructures, including those composed of unoriented, oriented and complexly oriented crystals. The orientation of spherulitic prismatic structures at the contact with the organic tube lining indicates that in the Recent species Crucigera websteri, C. zygophora, Floriprotis sabiuraensis, Pyrgopolon ctenactis and Spiraserpula caribensis, they grew towards the tube
lumen (Fig. 1.3-5 ![]() ). And a simple prismatic structure in Placostegus tridentatus and Vitreotubus digeronimoi probably indicates a similar direction of
growth. In fossil Hydroides sp. (Late Serravallian, Miocene, Deutsch Altenburg, Austria), an inner spherulitic prismatic layer shows a similar
direction (Fig. 1.6
). And a simple prismatic structure in Placostegus tridentatus and Vitreotubus digeronimoi probably indicates a similar direction of
growth. In fossil Hydroides sp. (Late Serravallian, Miocene, Deutsch Altenburg, Austria), an inner spherulitic prismatic layer shows a similar
direction (Fig. 1.6 ![]() ).
).
Click on thumbnail to enlarge the image.
Figure 1: 1, Inner organic lining of Spirobranchus giganteus showing organic sheets and fibres. 2, Josephella marenzelleri organic inner tube lining (orgl) in longitudinal section broken from the mineral part of the tube wall (arrows). 3, Crucigera websteri spherulitic prismatic inner tube layer in longitudinal section. 4, C. zygophora spherulitic prismatic inner tube layer in cross section. 5, Floriprotis sabiuraensis spherulitic prismatic inner tube layer (sphp) in longitudinal section. 6, Hydroides sp. spherulitic prismatic inner tube layer (sphp) in longitudinal section. Late Serravallian (Miocene) Deutsch Altenburg, Austria.
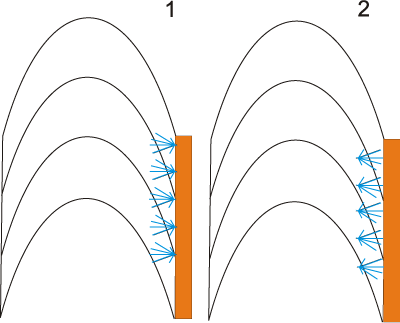
In support the hypothesis that the organic inner tube lining of serpulids serves as a nucleation site for crystals, their direction of growth should be away from the tube's lining
- lumen - (Fig. 2.1 ![]() ). Unoriented serpulid tube ultrastructures are most commonly found in contact with the organic inner tube lining. The morphology of these structures does not indicate the direction in which the crystal accreted. Similarly, lamello-fibrillar structures do not indicate the direction at which they grew. In contrast, the thicker ends of bundles of the spherulitic prismatic structure are always oriented in the direction of growth.
). Unoriented serpulid tube ultrastructures are most commonly found in contact with the organic inner tube lining. The morphology of these structures does not indicate the direction in which the crystal accreted. Similarly, lamello-fibrillar structures do not indicate the direction at which they grew. In contrast, the thicker ends of bundles of the spherulitic prismatic structure are always oriented in the direction of growth.
Click on thumbnail to enlarge the image.
Figure 2: 1, schematic line drawing showing spherulitic prismatic structure (blue) in longitudinal section with orientation supporting crystal nucleation from the inner organic lining (orange). 2, schematic line drawing showing spherulitic prismatic structure (blue) in longitudinal section with an orientation not supporting crystal nucleation from the inner organic lining (orange). Found in: Crucigera websteri, C. zygophora, Floriprotis sabiuraensis, Pyrgopolon ctenactis and Spiraserpula caribensis.
If the organic tube lining is used as a scaffold for the biomineralization of CaCO3, it is logical to assume that the mineralization should start on the organic lining and then grow away from it toward the periphery of the tube
wall (Fig. 2.1 ![]() ). If so, spherulitic prismatic structures in contact with the organic inner tube lining to should be oriented accordingly. However, in Crucigera websteri, C. zygophora, Floriprotis sabiuraensis, Pyrgopolon ctenactis and Spiraserpula caribensis, the spherulitic prismatic structure grows in the opposite direction, toward the organic portion of the inner tube
lining (Figs. 1
). If so, spherulitic prismatic structures in contact with the organic inner tube lining to should be oriented accordingly. However, in Crucigera websteri, C. zygophora, Floriprotis sabiuraensis, Pyrgopolon ctenactis and Spiraserpula caribensis, the spherulitic prismatic structure grows in the opposite direction, toward the organic portion of the inner tube
lining (Figs. 1 ![]() - 2.2
- 2.2 ![]() ). This direction of growth strongly suggests that the organic inner tube lining was formed after deposition of the innermost part of the mineral tube lining the wall. Either the organic tube is not used as a nucleation site in serpulids, or it may serve as such in species as yet unstudied and in species with an innermost lining of the tube wall built of crystals that do not indicate a direction of growth. If the organic tube lining of serpulids is not used as a scaffold for the biomineralization of CaCO3, then the question arises: Why does the organic tube have chemical properties that initiate nucleation of crystals? One possible explanation is that the inner tube lining is actually the terminations of organic sheets present between the chevron shaped growth lamellae that are laid down in the tube's lumen. These organic sheets in the tube wall of serpulids probably take an active part in the process of biomineralization and have the required chemical properties. In addition, biomineralization of the initial juvenile tube probably takes place on the initial organic layer. Alternatively, the organic inner tube lining may be secreted after the mineral is deposited. However, this does not explain the presence on outer portions of the organic lining of chemicals that can initiate biomineralization.
). This direction of growth strongly suggests that the organic inner tube lining was formed after deposition of the innermost part of the mineral tube lining the wall. Either the organic tube is not used as a nucleation site in serpulids, or it may serve as such in species as yet unstudied and in species with an innermost lining of the tube wall built of crystals that do not indicate a direction of growth. If the organic tube lining of serpulids is not used as a scaffold for the biomineralization of CaCO3, then the question arises: Why does the organic tube have chemical properties that initiate nucleation of crystals? One possible explanation is that the inner tube lining is actually the terminations of organic sheets present between the chevron shaped growth lamellae that are laid down in the tube's lumen. These organic sheets in the tube wall of serpulids probably take an active part in the process of biomineralization and have the required chemical properties. In addition, biomineralization of the initial juvenile tube probably takes place on the initial organic layer. Alternatively, the organic inner tube lining may be secreted after the mineral is deposited. However, this does not explain the presence on outer portions of the organic lining of chemicals that can initiate biomineralization.
In addition to a possible involvement in biomineralization, the organic lining could protect the worm against clefts in the calcareous wall of the tube.
If in some serpulids the organic lining is a site for the nucleation of minerals, its employment for biomineralization would be analogous to the systems of molluscs ( & , 1969; & , 1989; , 2000), brachiopods (, 1968; & , 1978) and bryozoans ( & , 1995; & , 2000), in all of which biomineralization of the shell is initiated at an organic layer. Further studies should determine whether or not the inner organic tube serves as a scaffold for the biomineralization of serpulid tubes.
The serpulid organic tube lining was either an evolutionary development from the organic tube of sabellids or evolved independently after biomineralized tubes in serpulids appeared in Middle Triassic times. However, if the organic tube linings of serpulids evolved directly from the organic tubes of sabellids, why is it that the calcareous sabellid (Glomerula) tube lacks an internal organic cuticle?
I acknowledge SYNTHESYS support under the European Commission's FPVI European funded Integrated Infrastructure Initiative to the projects NL-TAF-111, AT-TAF-611, SE-TAF-113, and SE-TAF-1520. I am grateful to Yannicke , Liz , Mark A. and Helmut for constructive remarks on the manuscript.
A.M., D.M. & K.M. (1985).- In vitro recalcification of organic matrix of scallop shell and serpulid tubes.- Journal of Molluscan Studies, Oxford, vol. 51, p. 284–289.
B.D. & J.D. (1973).- Generic and environmental control of carbonate mineralogy in serpulid (polychaete) tubes.- Journal of Geology, Chicago, vol. 81, p. 363-373.
A. (2000).- A new model for periostracum and shell formation in Unionidae (Bivalvia, Mollusca).- Tissue & Cell, Edinburgh, vol. 32, p. 405–416.
R., B. & J. (2000).- Organomineralization of cirratulid annelid tubes - fossil and recent examples.- Facies, Erlangen, vol. 42, p. 35-50.
R.H. (1958).- Tube formation by Pomatoceros triqueter (Polychaeta).- Journal of the Marine Biological Association of the United Kingdom, London, vol. 37, p. 315–322.
H.A. ten & E.K. (2009).- Taxonomy of Serpulidae (Annelida, Polychaeta): the state of affairs.- Zootaxa,
Auckland, vol. 2036, 126 p.
http://www.mapress.com/zootaxa/2009/f/zt02036p126.pdf
H.A. & S. (1989).- On biomineralization.- Oxford University Press, New York, 336 p.
E. (1993).- On the internal structure of calcified tube walls in Serpulidae and Spirorbidae (Annelida, Polychaeta).- Marine Fouling (Fuchaku Seibutsu Kenkyu), Iwate, vol. 10, n° 1, p. 17–20.
A.E., N., R.M.A., C.J. & G.C. (2010).- Insights into the composition, morphology, and formation of the calcareous shell of the serpulid Hydroides dianthus.- Journal of Structural Biology, San Diego, vol. 169, p. 145-160.
J.D. & W.J. (1969).- The influence of the periostracum on the shell structure of bivalve molluscs.- Calcified Tissue Research, New York, vol. 3, p. 274–283.
P.D. & M.J. (2000).- Skeletal ultrastructure and phylogeny of cyclostome bryozoans.- Zoological Journal of the Linnean Society, London, vol. 128, p. 337–399.
P.D., O., A. & J.W. (2010).- Raman spectroscopic study of the mineral composition of cirratulid tubes (Annelida, Polychaeta).- Journal of Structural Biology, San Diego, vol. 171, p. 402-405.
O. & H. (2009).- Calcareous tubeworms of the Phanerozoic.- Estonian Journal of Earth Sciences, Tallinn, vol. 58, p. 286-296.
O., H.A. ten & H. (2008a).- On the tube ultrastructure and origin of calcification in sabellids (Annelida, Polychaeta).- Palaeontology, London, vol. 51, p. 295–301.
O., H.A. ten, H. & K. (2008b).- Ultrastructure and mineral composition of serpulid tubes (Polychaeta, Annelida).- Zoological Journal of the Linnean Society, London, vol. 154, p. 633–650.
M.J. & P.D. (1995).- Calcitic nacreous ultrastructures in bryozoans: Implications for comparative biomineralization of lophophorates and molluscs.- Biological Bulletin, Woods Hole, vol. 188, p. 281-292.
S. & P.M. (2003).- An overview of biomineralization processes and the problem of the vital effect. In: P.M., J.J. & S. (eds.), Biomineralization.- Reviews in Mineralogy and Geochemistry, Saint Louis, vol. 54, p. 1–29.
A. (1968).- Significance of the structure of the brachiopod periostracum.- Nature, London, vol. 218, p. 551-554.
A. & S. (1978).- Secretion and ultrastructure of the periostracum of some terebratulide brachiopods.- Proceedings of the Royal Society of London, (Series B, Biological Sciences), vol. 202, p. 191-209.