◄ Carnets Geol. 15 (5) ►
Contents
[1. Introduction]
[2. Material and methods]
[3. Results]
[4. Discussion and conclusion]
[5. Systematics] [Bibliographic references] [Plate] and ... [Tables]
Department of Biological, Geological and Environmental
Sciences - Earth Sciences Section, Palaeoecological Research
Group, University of Catania, Corso Italia 55, I-95129 Catania (Italy)
Department of Biological, Geological and Environmental Sciences - Earth Sciences Section, Palaeoecological Research Group, University of Catania, Corso Italia 55, I-95129 Catania (Italy)
Published online in final form (pdf) on February 28, 2015
[Editor: Christian C. ;
technical editor: Bruno ; language editor: Stephen ]
Faunistic associations of the Lower Pleistocene sediments, out-cropping at Cartiera Molino along the true right bank of the Ippari River (Vittoria, SE Sicily), have been investigated. This study integrates data obtained from the analysis of ostracods, foraminifers, bryozoans and serpulids found within a six metre thick sedimentary section. This multiproxy approach allowed us to reconstruct the palaeoenvironmental evolution of this south-western sector of the Hyblean Plateau (Comiso-Vittoria area) from fluvially-influenced shallow marine settings, recorded in the lower portion of the succession, to progressively shallower, transitional and brackish environments, testified in mid levels, up to freshwater environments at the top of the section.
Palaeoenvironmental evolution; marine environment; brackish environment; freshwater environment; benthos; Pleistocene; Sicily.
F., A., (2015).- New faunistic data on the Pleistocene environmental evolution of the south-western edge of the Hyblean Plateau (SE Sicily).- Carnets Géol., Madrid, vol. 15, nº 5, p. 41-57.
Evoluzione ambientale pleistocenica nel settore sud-occidentale del Plateau ibleo (Sicilia SE).- Sono state studiate le associazioni ad ostracodi, foraminiferi, briozoi e serpulidi riscontrate in una successione sedimentaria pleistocenica, affiorante in località "Cartiera Molino” nei pressi di Vittoria (RG), lungo la riva destra del Fiume Ippari. L'analisi faunistica effettuata lungo la sezione ha consentito di definire l'evoluzione pleistocenica del settore sud-occidentale del Plateau Ibleo (area di Comiso-Vittoria) da paleoambienti marini del piano infralitorale, testimoniati nei livelli basali della successione, ad ambienti progressivamente meno profondi di transizione e salmastri, testimoniati nei livelli intermedi, fino ad ambienti francamente dulcicoli individuati nei livelli sommitali della sezione.
Evoluzione paleoambientale; ambiente marino; ambiente lagunare; ambiente lacustre; benthos; Pleistocene; Sicilia.
Nouvelles données faunistiques sur l'évolution environmentale pléistocène de la bordure sud-ouest du Plateau Hybléen (Sud-Est de la Sicile).- Nous avons étudié les associations d'ostracodes, de foraminifères, de bryozoaires et de serpulidés d'une succession sédimentaire d'âge Pléistocène inférieur qui affleure sur la rive droite de la rivière Ippari, à "Cartiera Molino" près de Vittoria (RG). L'analyse a permis de définir l'évolution pléistocène d'un secteur sud-ouest du Plateau Hybléen (secteur de Comiso-Vittoria ) depuis un milieu marin peu profond accusant quelques influences fluviatiles, observé à la base de la succession, en passant par des environnements progressivement moins profonds, de transition et puis saumâtres, tels qu'enregistrés dans les niveaux médians, jusqu'aux environnements d'eau douce, rencontrés au sommet de la section.
Évolution paléoenvironmentale ; milieu marin ; milieu lagunaire ; milieu d'eaux douces ; benthos ; Pléistocène ; Sicile.
The ecological distribution of marine benthic organisms depends not only on biotic factors, but also on several environmental physico-chemical and climatic parameters. In coastal-deltaic environments, ostracod distribution depends almost exclusively on salinity ( et al., 1972; et al., 2000; & , 2002, inter alias), while in fully marine environments depends on several other factors largely varying with depth, such as temperature, texture of the bottom sediments and local hydrodynamic energy, availability of food and oxygen. Consequently, particular ostracod associations can be considered as indicative of specific environmental conditions and used as tools to reconstruct palaeoenvironments.
Among the benthic organisms employed to define environmental and palaeoenvironmental conditions, ostracods have been used for a long time in various geological research fields, particularly in oceanographic (, 1984, inter alias) and ecological studies ( & , 1984; , 1987; & , 1989, inter alias). These organisms have been shown to be particularly important for these purposes in deep-marine environments ( et al., 1997; & , 2001, inter alias) as well as in shelf, marginal and non marine environments.
Indeed, like a few other taxonomic groups, ostracods exhibit a wide range of ecological adaptations and include typically marine, brackish and freshwater species allowing the evolution from marine to transitional and/or continental environments to be traced ( & , 2005; & , 2008; & , 2008; et al., 2011, inter alias). Ostracods are a useful tool for palaeoenvironmental interpretation in addition to other marine benthic faunistic groups in a multidisciplinary study performed by et al. (2009) on a section from the Pleistocene Barcellona Pozzo di Gotto Basin (NE Sicily). In that study, data provided from several benthic organisms (molluscs, bryozoans, serpuloideans, crustaceans, foraminifers) were integrated with sedimentological, taphonomic, and biostratigraphic observations.
In
the present study, a similar methodological approach is adopted to detail the palaeobasin
evolution recorded in a Pleistocene sedimentary succession out-cropping along
the south-western edge of the Hyblean Plateau, SE Sicily
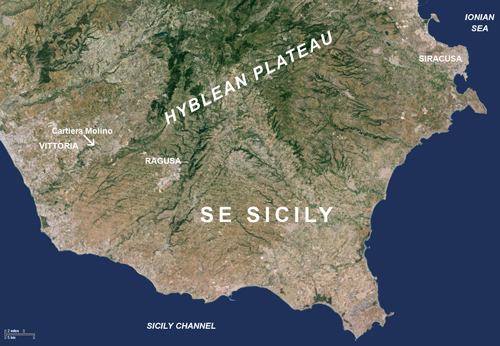
(Fig. 1 ![]() ).
).
Click on thumbnail to enlarge the image.
Figure 1:
Geographical location of the Cartiera Molino section in South-Eastern
Sicily.
[Some rights reserved: Imagery © 2015 TerraMetrics, Map Data © 2015 Google].
Editorial note: "The authors are the 'sole responsible' for the usage made
of texts, illustrations (tables and drawings), photos and videos provided and
used in their respective publications."
The
present analysis focuses on a sedimentary succession, six metres thick (Figs. 1 ![]() ,
2
,
2 ![]() & 3
& 3 ![]() ), cropping out at Cartiera
Molino (F. 276, IV NW; Lat. 36°56'58";
Long. 2°07'00") along the true right bank of the Ippari River, near
Vittoria (RG). In this area the Quaternary sedimentary succession lies
unconformably on Miocene carbonate formations and/or on Lower Pliocene
calcareous marls, locally known as "Trubi" (Figs. 1
), cropping out at Cartiera
Molino (F. 276, IV NW; Lat. 36°56'58";
Long. 2°07'00") along the true right bank of the Ippari River, near
Vittoria (RG). In this area the Quaternary sedimentary succession lies
unconformably on Miocene carbonate formations and/or on Lower Pliocene
calcareous marls, locally known as "Trubi" (Figs. 1 ![]() - 2
- 2 ![]() ). Pleistocene sediments
consist predominantly of yellow calcareous sands, sands and silts and/or
calcarenites with Arctica islandica (,
1767) and Hyalinea balthica (,
1783) evolving laterally and upward to marine whitish silts and sands locally
capped by lacustrine white calcareous silts and travertine deposits, which
locally show discontinuous breccias and conglomerates intercalations. This
succession is truncated by an erosive surface, on which early-mid Pleistocene
sandy sediments are present. This sedimentary succession represents the
transition from the marine whitish silts and sands to the white calcareous silts
and continental travertines.
). Pleistocene sediments
consist predominantly of yellow calcareous sands, sands and silts and/or
calcarenites with Arctica islandica (,
1767) and Hyalinea balthica (,
1783) evolving laterally and upward to marine whitish silts and sands locally
capped by lacustrine white calcareous silts and travertine deposits, which
locally show discontinuous breccias and conglomerates intercalations. This
succession is truncated by an erosive surface, on which early-mid Pleistocene
sandy sediments are present. This sedimentary succession represents the
transition from the marine whitish silts and sands to the white calcareous silts
and continental travertines.
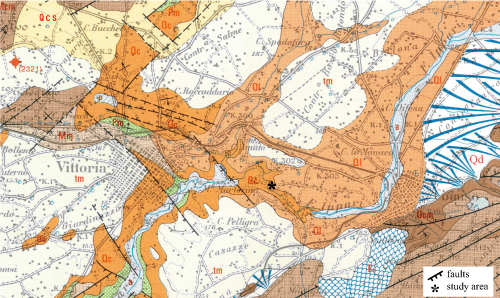
Click on thumbnail to enlarge the image.
Figure 2: Geological map of the Cartiera Molino section area (excerpt from et al., 1984, Carta geologica della Sicilia SE alla scala 1:100.000, modified). a) Alluvium. Recent; L) Landslide; Qd) Detritus deposits; tm) Sands, calcarenites and marine terraces. Middle-Upper Pleistocene; Ql) Silts and travertine, Qc) calcarenites, Qcs) sands and clays. Lower Pleistocene; Pm) Calcareous marls of Trubi. Lower Pliocene; Mn) Mudstones and marls of Tellaro Fm. Upper Miocene; Mcm) Calcarenites of Ragusa Fm (Irminio Member). Lower Miocene; Ocm) Calcarenites of Ragusa Fm (Leonardo Member). Upper Oligocene.
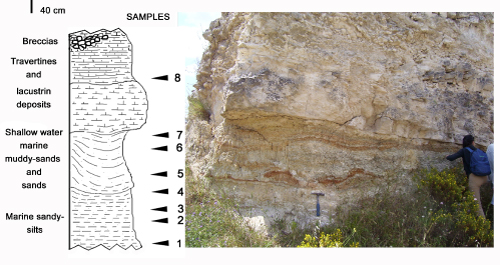
Click on thumbnail to enlarge the image.
Figure 3: The sedimentary succession cropping out at Cartiera Molino and the corresponding stratigraphical log with sampling location.
The white calcareous silts of the Cartiera Molino section were described in a short note by (1961), who recorded some freshwater ostracods, and in a geological study by et al. (1979) reporting the presence of the freshwater gastropods Bithynia leachi (), Planorbis planorbis () and Lymnaea peregra ().
The molluscan faunas were susequently studied by (1989), who indicated the presence of shallow-water marine species among which Flexopecten hyalinus (, 1795), Loripinus fragilis (, 1836), Loripes lacteus (, 1758), Cerithium vulgatum , 1792, Conus mediterraneus (, 1791) and Bittium reticulatum (, 1778).
Due to the lack of species stratigraphically significant, these sediments have been referred to the early Pleistocene on the basis of their stratigraphic position. Indeed, they overlay calcarenites containing A. islandica () and H. balthica ().
The
examined section (Fig. 3 ![]() ) exposes at the base 1.5 metres of sandy silts followed
by 1.5 m of more or less cemented muddy sands and sands that are capped by about
3 metres of travertine precipitates. Within travertines a 45 centimetres thick
layer of whitish carbonate limestone is interbedded, at 45 centimetres from its
wavy basal contact.
) exposes at the base 1.5 metres of sandy silts followed
by 1.5 m of more or less cemented muddy sands and sands that are capped by about
3 metres of travertine precipitates. Within travertines a 45 centimetres thick
layer of whitish carbonate limestone is interbedded, at 45 centimetres from its
wavy basal contact.
For
this study, a total of eight samples was collected (Fig. 3 ![]() ) with samples 1-3,
coming from the sandy silts from the basal part of the section, samples 4-6 from
sands in the intermediate part of the section, and samples 7-8 from the
whitish limestones.
) with samples 1-3,
coming from the sandy silts from the basal part of the section, samples 4-6 from
sands in the intermediate part of the section, and samples 7-8 from the
whitish limestones.
From each sample 300 cc of sediment was washed routinely, as reported in (2003, 2005). All specimens of ostracods, foraminifers, bryozoans and serpuloideans were picked from the > 63 µm fraction. They were determined to species level when possible. Ostracod species were particularly examined (see below in the taxonomic section) and their specimens measured under a stereo microscope and some photographed using an LMU Tescan Vega II Scanning Electron Microscope. The fossil material is housed at the Paleontological Museum of the University of Catania (PMC).
In
the ostracod fauna, 41 species, belonging to 27 genera, have been found (Table 1, Pl. 1 ![]() ). A gradual decrease in the number of both species and genera from the
lower part of the section to the top has been observed. Benthic foraminifers are
present with a total of 15 species (Table 2, Fig. 4
). A gradual decrease in the number of both species and genera from the
lower part of the section to the top has been observed. Benthic foraminifers are
present with a total of 15 species (Table 2, Fig. 4 ![]() ). They are common and
diverse in the basal samples, decrease in the central part of the section and
disappear in the upper part. Serpuloideans and bryozoans (Tables 3 - 4, Fig.
5
). They are common and
diverse in the basal samples, decrease in the central part of the section and
disappear in the upper part. Serpuloideans and bryozoans (Tables 3 - 4, Fig.
5 ![]() ) are
represented by a total of 8 and 10 species, respectively. They occur mostly in
samples from the basal part of the section, sporadically in the middle part and
are completely absent from the top part.
) are
represented by a total of 8 and 10 species, respectively. They occur mostly in
samples from the basal part of the section, sporadically in the middle part and
are completely absent from the top part.

Click on thumbnail to enlarge the image.
Figure 4: Foraminifers. A) Rosalina globularis , 1826. Scale bar 200 µm; B) Ammonia beccarii (, 1758). Scale bar 200 µm; C) Cibicides lobatulus ( & , 1798). Scale bar 200 µm; D) Elphidium sp. Scale bar 200 µm; E) Asterigerinata mamilla (, 1858). Scale bar 200 µm; F) Elphidium aculeatum (, 1846). Scale bar 200 µm; G) Elphidium sp. (teratologic specimen). Scale bar 250 µm; H) Elphidium sp. (teratologic specimen). Scale bar 250 µm.
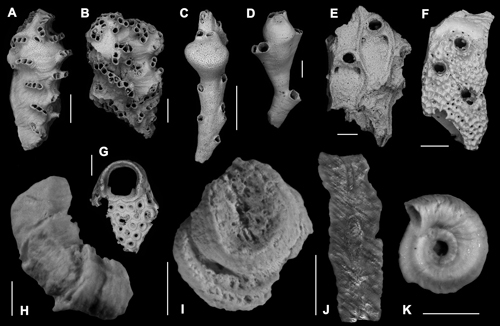
Click on thumbnail to enlarge the image.
Figure 5: Serpuloideans, bryozoans and bioimmuration casts. A) Platonea stoechas , 1976. Scale bar 500 µm; B) ? Tubulipora plumosa , 1898. Scale bar 500 µm; C) ? Crisia pyrula , 1990. Scale bar 500 µm; D) Crisia fistulosa (, 1867). Scale bar 200 µm; E) Calpensia nobilis (, 1796). Scale bar 200 µm; F) Schizomavella sp. Scale bar 200 µm; G) ? Watersipora sp. Scale bar 200 µm; H) Vermiliopsis striaticeps (, 1862). Scale bar 500 µm; I) Spirobranchus polytrema (, 1844). Scale bar 1 mm; J) Undersurface of an encrusting portion of a serpulid skeleton exposing the mould of a Posidonia leaf. Scale bar 500 µm; K) Neodexiospira pseudocorrugata (, 1905). Scale bar 500 µm.
The
basal sandy-silty layers (samples 1-3) contain abundant and diversified benthic
associations. Ostracods range from 14 to 23 species per sample. The most
abundant species are Aurila gr. A.
convexa (, 1850), A. cf. A. cruciata (, 1950), Urocythereis sororcula (, 1880), Loxoconcha
gibberosa , 1878, Neonesidea
mediterranea (, 1894), Costa
batei (, 1880), Urocythereis
margaritifera (, 1894), Graptocythere
hscripta (, 1900) and Carinocythereis
whitei (, 1850). Benthic foraminifers are common with 4-7
species per sample. Associations are dominated by Ammonia beccarii
(, 1758) (Fig. 4.B ![]() ),
Rosalina globularis
,
1826, Cancris auriculus (
& , 1798), Asterigerinata mamilla (,
1858) (Fig. 4.E
),
Rosalina globularis
,
1826, Cancris auriculus (
& , 1798), Asterigerinata mamilla (,
1858) (Fig. 4.E ![]() ), Cibicides lobatulus
( & , 1798)
(Fig. 4.C
), Cibicides lobatulus
( & , 1798)
(Fig. 4.C ![]() ) and
by some species belonging to Elphidium (Fig. 4.F-H
) and
by some species belonging to Elphidium (Fig. 4.F-H ![]() ).
).
Serpuloideans
and bryozoans are relatively abundant and diversified, although extremely
fragmented. Serpuloideans are usually found as less than 1mm tube fragments and
bryozoans consist of no more than 2-4 associated zooids (Fig.
5.E-F ![]() ) and even
single broken zooids (Fig. 5.G
) and even
single broken zooids (Fig. 5.G ![]() ). The internodes of jointed species (Crisia
spp.) are broken (Fig. 5.C-D
). The internodes of jointed species (Crisia
spp.) are broken (Fig. 5.C-D ![]() ) and exceptionally a few larger cyclostome bryozoan
colony fragments (Fig. 5.A-B
) and exceptionally a few larger cyclostome bryozoan
colony fragments (Fig. 5.A-B ![]() ) have been found. Dissolution is often evident. For
these reasons several specimens were identified to species level, or even to
genus level, only tentatively.
) have been found. Dissolution is often evident. For
these reasons several specimens were identified to species level, or even to
genus level, only tentatively.
Serpuloideans
include 9 species, but they are all present in sample 3 whereas they are absent
in sample 1 and represented by a single species in sample 2. Specimens belonging
to the genus Hydroides, particularly
to H. dianthus (, 1873)
and H. elegans (, 1883),
as well as to the species Vermiliopsis
striaticeps (, 1862) (Fig.
5.H ![]() ). Other species, such as Serpula
vermicularis , 1767, Neodexiospira
pseudocorrugata (, 1905) and Janua
pagenstecheri (, 1866) are subordinate.
). Other species, such as Serpula
vermicularis , 1767, Neodexiospira
pseudocorrugata (, 1905) and Janua
pagenstecheri (, 1866) are subordinate.
Bryozoans
total 10 species. Species richness per sample ranges from 4 to 9. Internodes of Crisia,
including those of the species C.
fistulosa (, 1867) (Fig.
5.D ![]() ) and ? C. pyrula ,
1990 (Fig. 5.C
) and ? C. pyrula ,
1990 (Fig. 5.C ![]() ) dominate the fossil
associations. Platonea stoechas ,
1976 (Fig. 5.A
) dominate the fossil
associations. Platonea stoechas ,
1976 (Fig. 5.A ![]() ) is also abundant whereas all other species are represented by
very few or even a single fragment, as is the case for ? Watersipora sp.
(Fig. 5.G
) is also abundant whereas all other species are represented by
very few or even a single fragment, as is the case for ? Watersipora sp.
(Fig. 5.G ![]() ).
).
Bioimmuration
casts have been observed on the basal surfaces of some encrusting specimens,
that were better preserved, belonging to both serpuloidean and bryozoans taxa (Fig.
5.J ![]() ).
).
Samples 4-6 from the muddy sands and sands, of the intermediate part of the section, contain benthic associations decidedly poorer than those of the basal part. Furthermore, both species richness and specimen abundance decrease markedly upward and fossil content is nearly completely absent in the topmost sample 6 (Tables 1 - 2 - 3 - 4). Ostracods are restricted to sample 4, where only 6 species occur, 5 of which share with associations from the previous samples. Three of them, namely Aurila gr. A. convexa (, 1850), A. cf. A. cruciata (, 1950) and Costa batei (, 1866), are abundant in the basal part, and still predominate.
Benthic foraminifers slightly reduce their diversity in samples 4-5 (6 species) and completely disappear in sample 6. Ammonia beccarii (, 1758), Cibicides lobatulus ( & , 1798) and Elphidium crispum (, 1758) are more frequent than other species, such as Asterigerinata mamilla (, 1858), Elphidium spp. Bolivina alata (, 1862), Bulimina marginata (, 1826) and Cassidulina carinata , 1896, are only present in sample 5. Serpuloideans and bryozoans are only sporadically present with a single species each, and very few specimens, in sample 4 or 5, whereas they are absent from sample 6.
Samples
7-8 are from the marly sediments collected from the top part of the section;
they contain only ostracods, whereas all other investigated groups are lacking. Ostracods
include a total of 7 species with a species richness ranging from 3 to 7. Ilyocypris
gibba (, 1808) (Pl. 1 ![]() ,
fig. U), I. monstrifica (, 1862) (Fig.
6.A
,
fig. U), I. monstrifica (, 1862) (Fig.
6.A ![]() ), Cypridopsis vidua (O.F. , 1776),
Eucypris virens
(, 1820) (Fig. 6.A
), Cypridopsis vidua (O.F. , 1776),
Eucypris virens
(, 1820) (Fig. 6.A ![]() ), Candona
neglecta , 1887, C. angulata ,
1900 (Pl. 1
), Candona
neglecta , 1887, C. angulata ,
1900 (Pl. 1 ![]() ,
figs. V & Z), and Herpetocypris
sp. have been detected. Rare
characean oogones (Fig. 6.C
,
figs. V & Z), and Herpetocypris
sp. have been detected. Rare
characean oogones (Fig. 6.C ![]() ) have been found in these samples.
) have been found in these samples.
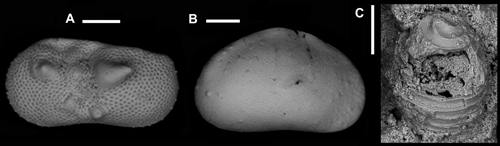
Click on thumbnail to enlarge the image.
Figure 6: Freshwater faunas and floras. A - Ilyocypris monstrifica (, 1862). LV external lateral view. Scale bar 200 µm; B) Eucypris virens (, 1820). RV external lateral view. Scale bar 200 µm; C) Characean oogone. Scale bar 250 µm.
Planktonic foraminifers are very rare along the section. In the basal samples (1-2) only three species have been recognized: Globigerinoides ruber (, 1839), Globigerinoides elongatus (, 1839) and Globorotalia inflata (, 1839). Samples 3-4 do not contain planctonic foraminifers. Sample 5 shows higher diversity with Globigerinella calida (, 1962), Neogloboquadrina pachyderma (, 1861), Globorotalia inflata (, 1839), Globigerinoides ruber (, 1839), Globigerinoides trilobus (, 1850) and Orbulina spp. The association is indicative of the early Pleistocene (Calabrian). Samples 6-8 bear only few planktonic species (Table 2) and the globorotaliids Globorotalia margaritae & , 1965, and G. puncticulata , 1832, reworked from the Lower Pliocene chalks (Trubi Formation).
Benthic associations from the lower part of the Cartiera Molino section (Samples 1-3) point to shallow-water marine habitats falling within the inner shelf and the infralittoral zone of the benthic zonation scheme proposed by and (1964) and successfully applied to the interpretation of palaeocommunities ( , 1985; et al., 1994, inter alias).
Particularly, the ostracod fauna characteristically consists of species thriving in Infralittoral environments to which a subordinate number of rare, more euryecious ubiquitous species add, whose distributions extends also to the circalittoral zone. This latter group includes both relatively common-to-abundant species belonging to the genera Aurila, Neonesidea, Loxoconcha, Carinocythereis and Paracytheridea, as well as rare species, such as Cytherella alvearium ( et al., 1975) and Tetracytherura angulosa () ( et al., 1998; & , 1987, inter alias).
Typically infralittoral species are Urocythereis sororcula (), U. margaritifera (), Costa batei (), Graptocythere hscripta (), Cytheretta spp., Caudites calceolatus (), Leptocythere lagunae and Semicytherura spp., that have been usually reported from many authors.
The presence of Cytherelloidea beckmanni - has a considerable palaeoenvironmental significance, as this species is characteristic of very shallow water environments (, 1987, inter alias). Analogously, Aurila arborescens () is a shallow marine species, characteristically associated to vegetate bottoms ( et al., 1989). Furthermore, A. arborescens can be found also in brackish lagoonal and estuarine environments. Consequently, the presence of this latter species, as well as of some taxa, such as Leptocythere lagunae, Semicytherura paradoxa and Loxoconcha spp. point to the possible (at least temporary) presence in the area of freshwater inputs. Indeed, all the above reported species are known as able to tolerate salinity fluctuations and to be also widely distributed in brackish waters ( & , 2002; & , 2005).
Therefore, ostracod association from this part of the section, indicate a shallow-water marine environment very close to the coast and possibly in the upper Infralittoral (probably less than 20 m deep) that was affected by freshwater river inputs, in agreement with the environmental assignment scheme proposed by & (1986), et al. (1998) and supported by the autoecological features of individuals species.
Benthic foraminifera associations concur to this hypothesis because most species (e.g., Ammonia beccarii, Elphidium crispum) are typical of inner shelf environments (, 1991).
Analogously, serpuloideans and bryozoans support the same inferences. Associations consist of typical infralittoral species. They are the serpulids V. striaticeps, Hydroides spp., S. polytrema and the spirorbids J. pagenstekeri and N. pseudocorrugata, with these latter two species more typical of the upper horizon of the infralittoral zone ( et al., 2013). Also several bryozoans, and particularly P. stoechas, C. fistulosa, Tubulipora spp. and C. nobilis are typical representatives of infralittoral habitats where they are often associated to soft algae and Posidonia oceanica meadows (, 1962; , 1976; , 1986). The euryecious ubiquitous species found, such as the serpulid S. vermicularis and the bryozoans C. pyrula (, 1990), have distributions including infralittoral habitats ( et al., 2013; , 1990). Last but not least, the serpulid H. elegans may indicate water with low salinity value (, 1981).
Furthermore,
some taphonomic features contribute to strengthen this environmental
attribution. Some bioimmuration evidences on the undersurfaces of encrusting
portions of serpulid (Fig. 5.J ![]() ) and bryozoan skeletons concur to testify to the
presence of algae and plants that probably represented the substratum for most
of the taxa recovered.
) and bryozoan skeletons concur to testify to the
presence of algae and plants that probably represented the substratum for most
of the taxa recovered.
This environmental attribution is in agreement with previous inferences obtained through malacofaunas that suggest shallow marine environments ranging from the Posidonia meadows assemblage (HP) to the Muddy-Sand Assemblages in Sheltered Areas (SVMC), up to a transition to the Euryhaline and Eurytherm Assemblage (LEE) sensu & (1964) and (1982), as suggested by (1980).
The palaeoenvironmental interpretation of the middle part of the section (samples 4-6) is less supported owing to the extremely scant fossils found in these layers. This is particularly apparent for the macroinvertebrates and subordinately for ostracods, but not for foraminifers, except in the uppermost layer.
Ostracod association from sample 4 consists exclusively of species that are typical infralittoral representatives and could therefore point to a palaeoenvironment roughly comparable to those from previous samples. Nevertheless, it is oligospecific and extremely impoverished when compared to associations from the underlying layers, although nearly all species from the association of sample 4 are shared with those from previous samples. The progressive decrease in richness observed along the section at both species and genus level could be interpreted as related to a progressive shallowing, following & (2002).
Of
special interest is also the relevant number of teratologic specimens of Elphidium
sp. (Fig. 4.G-H ![]() ) whose occurrence could indicate environmental stress (
et al., 1998; et
al., 2000; & ,
2001) that may be caused by lower
salinity induced by freshwater inputs ( et
al., 2005).
) whose occurrence could indicate environmental stress (
et al., 1998; et
al., 2000; & ,
2001) that may be caused by lower
salinity induced by freshwater inputs ( et
al., 2005).
Although a certain similarity in inferred palaeoenvironments for associations from the lower and central part of the sections, the strong oligotypy of the latter ones point to strong changes in palaeoenvironmental features and parameters and particularly to stressed conditions (AAA). In the present instance a possible explanation for the progressive reduction of taxa could have been caused by the increase of freshwater inputs, already present but probably only temporarily active in previous times. An additional source of disturbance can be foreseen in the occurrence of high sedimentation episodes consistent with a fluviatile input. Local/temporary high sedimentation events are also suggested by the occurrence of discontinuous layers of breccias and conglomerate intercalations in corresponding layers, from neighbouring areas.
In Sample 5 the association is composed of species living in lagoons and /or inner shelf environments (e.g., Elphidium, Ammonia, Cibicides) together with Bulimina marginata, Bolivina alata and Cassidulina laevigata. These species are present in the Atlantic Ocean and Mediterranean Sea in mud and muddy sandy substrata at variable depth from few tens to hundreds meters. The presence of these taxa, the increasing diversity and the lithological features suggests an upward increasing content in mud within a shallow, fluvial-influenced marine setting.
Fossil
associations from the highest stratigraphic levels of the succession (samples
7-8) point to a lacustrine palaeoenvironment or, at least, to the inner confined
portion of a lagoon. This hypothesis is supported by the absence of any taxon
thriving in marine environments. Serpuloideans and bryozoans, as well as
foraminifers are completely absent. Only ostracods occur but none of the species
found in the associations from these layers are shared with those of the
underlying layers. Associations are relatively diversified and exclusively
consist of species that presently live within lakes, ponds and canals, such as C.
vidua, E. virens, I. gibba and I. monstrifica (,
2002, inter alias) or whose distribution extends also to inner lagoons
and other brackish coastal waters, such as C. angulata and C. neglecta
(AA). The recovery of oogones of characeans (Fig. 6.C ![]() ), plants specially adapted
to colonise freshwater settings (
et al., 2006),
is consistent with the above inferences.
), plants specially adapted
to colonise freshwater settings (
et al., 2006),
is consistent with the above inferences.
In these continental basins a large amount of sediments, coming from the erosion of the neighbouring Pliocene outcrops (Trubi), was deposited as demonstrated by the finding of specimens of Globorotalia margaritae and G. puncticulata.
The Cartiera Molino section, with its sediments and the palaeontological content, testifies to the transition from marine shallow water settings to lagoon environments up to continental lacustrine environments, contributing information to a better understanding of the evolution of the western sector of the Hyblean Plateau.
Some species of ostracods found in the stratigraphic section of Cartiera Molino, that are rare and/or little known from Sicily are briefly commented below. They are mostly freshwater taxa, and among the less reported as fossils owing to the rare preservation in these palaeoenvironments.
Class Ostracoda , 1806
Order Platycopida , 1866
Family Cytherellidae , 1866
Genus Cytherelloidea , 1929
Cytherelloidea beckmanni , 1971
(Pl. 1 ![]() , fig. A)
, fig. A)
1971 Cytherelloidea beckmanni , p. 262, Pl. 2, figs. 1c, 2c, 3c; Pl. 45, figs. 14-15;
1972 Cytherelloidea beckmanni : , p. 72, Pl. 2, fig. 3;
1975 Cytherelloidea beckmanni : & , p. 2, Pl. 1, fig. 7;
1987 Cytherelloidea beckmanni : , p. 45, Pl. 1, figs. 1-2;
1997 Cytherelloidea beckmanni : , p. 74. Pl. 1, fig. 1;
2005 Cytherelloidea beckmanni : et al., p. 74.
Remarks: This species is known from the Miocene (, 1987) to the Recent (, 1997). In the Recent, C. beckmanni , as well as other species of the genus Cytherelloidea are considered to be characteristic of very shallow-water environments ( & , 1975; , 1987; & , 1989). Consequently, the finding of this species as fossil could be confidently used as a good palaeoenvironmental indicator. Few specimens of this species were found, restricted to the lower levels of the section (samples 1-2).
Order Podocopida , 1866
Family Hemicytheridae , 1953
Genus Aurila , 1955
Aurila arborescens (, 1865)
(Pl. 1 ![]() , fig. L)
, fig. L)
1865 Cythere arborescens , p. 190, Pl. 9, figs. 5-8;
1868 Cythere woodwardii , p. 93, Pl. 10, figs. 19-21;
1963 Aurila woodwardii (): , p. 8, Pl. 1, figs. 1-3;
1975 Aurila woodwardii (): , & , p. 44, Pl. 20, figs. 8-11;
1975 Aurila woodwardii (): , p. 30;
1985 Aurila arborescens (): , & , p. 156, Pl. 1, figs. 5-8; Pl. 2, figs. 1-4;
1989 Aurila arborescens (): , & , p. 158, Fig. 63; Pl. 4, fig.10;
2005 Aurila arborescens (): et al., p. 93, Pl. 1, figs. 11-12.
Remarks - This species is known from the late Pliocene of Forlì (, 1975) and of NW France and Cornwall ( et al., 1985). It has been also reported from the present-day Mediterranean Sea ( et al., 1975), the SW Wales and in the Thames estuary ( et al., 1989). Presently, A. arborescens is a shallow marine phytal species, found also in brackish lagoonal and environments. Only a few specimens were found in sample 3.
Suborder Cypridocopina , 1901
Superfamily Cypridoidea , 1845
Family Candonidae , 1900
Subfamily Candoninae , 1900
Genus Candona , 1845
Candona neglecta , 1887
1887 Candona neglecta , p. 279, Pl. 15, figs. 5-7; Pl. 19;
1900 Candona neglecta : , p. 17;
1957 Candona neglecta : , p. 21, Pl. 3 figs 1-5;
1998 Candona (Candona) neglecta : & , p. 78, Pl. 1, fig. E;
2000 Candona neglecta : , p. 77, Fig. 26;
2003 Candona neglecta : & , p. 15, Fig. 2;
2006 Candona neglecta : , p. 331;
2008 Candona neglecta : et al., p. 13, Pl. 2, fig. 1.
Remarks - C. neglecta is presently distributed throughout the Holarctic biogeographical region (, 2000; & , 2003), where it has a long and nearly continuous stratigraphic record. The earliest occurrence of the C. neglecta group is from the Late Cretaceus of Mongolia ( & 2003). In the Recent the species is widespread in all permanent or temporary freshwater habitats, such as lakes, rivers, deltaic settings, springs and streams but, being able to live in waters with salinities ranging from 0.5 to 16 ‰, it also colonises brackish waters. Nevertheless, it is rare in such environments. C. neglecta prefers cool waters and tolerates low oxygen content in the water.
The species was not known as fossil from Sicily, whereas it probably thrives in present-day lakes. Specimens of Candona lindneri , 1969, have been recorded as living from this region by et al. (2006). This latter species has been distinguished from C. neglecta , owing to the presence of tubercles and spines, but its validity and distinction from C. neglecta has been questioned by & (2003).
Several valves of C. neglecta were found in sample 7.
Candona angulata , 1900
(Pl. 1 ![]() ,
figs. V & Z)
,
figs. V & Z)
1900 Candona angulata , p. 18, Pl. 1, figs. 1-17.
1963 Candona angulata : , p. 76, Pl. 3, figs. 1-8.
1996 Candona angulata : & , p. 40.
2011 Candona angulata : -, p. 69.
Remarks - This species lives in coastal ponds and occasionally in brackish lagoons (, 1990). Candona angulata has already been reported in the Pleistocene of Sicily by (1963). Unlike the previous species, it has not yet been reported from present-day freshwater environments of this region.
Family Cyprididae , 1845
Genus Eucypris , 1891
Eucypris virens (, 1820)
(Fig. 4.B ![]() )
)
1820 Monoculus virens , p. 174, Pl. 18, figs. 15, 16.
1900 Cypris virens (): , p. 62, Pl. 15, figs. 1-4, 7-10, 16-18.
1996 Eucypris virens (, 1820): & , p. 148, Pl. 1, fig. 16.
2006 Eucypris virens (, 1820): et al., p. 5.
2013 Eucypris virens (, 1820): et al., p. 4
Remarks - E. virens lives in ponds and even temporary pools (, 1990). This species was already been reported from the Recent of Sicily by et al. (2006). It is here reported as fossil for the first time from this region.
Family Ilyocyprididae , 1900
Subfamily Ilyocypridinae , 1900
Genus Ilyocypris & , 1889
Ilyocypris gibba (, 1808)
(Pl. 1 ![]() , fig. U)
, fig. U)
1808 Cypris gibba , p. 91, Pl. 3, figs. 13-14, 17;
1965 Ilyocypris gibba (): , p. 345, Fig. 50;
1979 Ilyocypris gibba (): & , p. 195, Pl. 1, fig. 2;
1998 Ilyocypris gibba (): & , p. 80, Pl. 2, fig. A;
1999 Ilyocypris gibba (): et al., p. 297, Pl. 2, fig. 5;
2000 Ilyocypris gibba (): , p. 245, Fig. 104;
2005 Ilyocypris gibba (): & , p. 40, Pl. 1, figs. 1-3, 7;
2006 Ilyocypris gibba (): et al., p. 124, Fig. 2 (I-K);
2006 Ilyocypris gibba (): et al., p. 5;
2008 Ilyocypris gibba (): , p. 109, Fig. 3;
2008 Ilyocypris gibba (): et al., p. 12, Pl. 1, figs. 10-11;
2013 Ilyocypris gibba (): et al., p. 4.
Remarks: I. gibba () is a Holarctic species, known from a very wide area in Europe and Asia, as well as from East Africa and North America (, 1990). In Sicily it has been recently recorded in Recent deposits by et al. (2006). The stratigraphical distribution of I. gibba () is wide, ranging from the Tortonian to the Recent ( et al., 2008). The species is widespread in all freshwater environments, in a wide temperature range.
Ilyocypris monstrifica (, 1862)
(Pl. 1 ![]() , fig. Z)
, fig. Z)
1862 Cypris monstrifica , p. 45, Pl. 3, figs. 4-5;
1970 Ilyocypris monstrifica (, 1862): , p. 109-110;
1988 Ilyocypris monstrifica (, 1862): , p. 153, Fig. 3;
2001 Ilyocypris monstrifica (, 1862): , p. 346;
2003 Ilyocypris monstrifica (, 1862): et al., p. 3;
2006 Ilyocypris monstrifica (, 1862): et al., p. 124;
2006 Ilyocypris monstrifica (, 1862): , p. 331;
2008 Ilyocypris monstrifica (, 1862): , p. 110;
2011 Ilyocypris monstrifica (, 1862): , p. 174.
Remarks: I. monstrifica () is widely distributed in Europe from lakes, canals and large rivers (, 2002). It has also been reported from the floodplain of the Chi River basin in Thailand (, 2011) and from Syria (, 2006). Nevertheless, this species has rarely been reported from Italy and the present record is the first one from Sicily.
The authors are grateful to the two referees and the editor for comments and suggestions and to Alfio Viola (University of Catania) for SEM assistance. Paper financially supported by Catania University PRA grants to A. . Catania Palaeoecological Research Group contribution nº 400.
D. (2008).- Differences in Ostracoda (Crustacea) assemblages between two maar lakes and one sinkhole lake in the Konja region of Turkey.- Turkish Journal of Zoology, Ankara, vol. 32, p. 107-113.
S. (2001).- The Ostracoda (Crustacea) Fauna of lake Erikli, Hamam, Mert, Pedina and Saka (Iğneada, Kirklareli, Turkey).- Turkish Journal of Zoology, Ankara, vol. 25, p. 343-355.
F.O., G., Di V., P., E. & D. (2000).- An integrated micropaleontological approach applied to Late Pleistocene-Holocene palaeoclimatic and palaeoenvironmental changes (Gaeta Bay, Tyrrhenian Sea). In: M.B., ed., Climates: Past and Present.- Geological Society, Special Publication , Bath, vol. 181, p. 95-111.
J.F. (1987).- Marine lower Pliocene Ostracoda of southern Spain with notes on the recent fauna.- Bulletin of the geological Institution of the University of Uppsala, (N.S.), vol. 13, p. 1-94.
J., D.J. & J.E. (1985).- G.S. 's Pleistocene ostracods from the Brickearth of the Nar Valley, Norfolk.- Journal of Micropalaeontology, London, vol. 4, nº 2, p. 153-158.
J., D.J. & J.E. (1989).- Marine and brackish water ostracods.- Synopses of the British Fauna, London, vol. 43, 343 p.
M., H., V. & K. (1997).- Benthonic ostracods and deep watermasses. A qualitative comparison of Southwest Pacific, Southern and Atlantic Oceans.- Palæogeography, Palæoclimatology, Palæoecology, vol. 131, p. 287-302.
J.-F. & F. (1984).- Importance du taxon générique chez les ostracodes fossiles.- Bulletin de la Société géologique de France, Paris, vol. 7, nº 4, p. 591-602.
P.I. (1971).- Die Ostracoden des Kustenbereiches von Naxos (Griechenland) und ihre Lebensberiche.- Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut, vol. 67, p. 55-356.
D. (1997).- The shallow- water marine ostracods of Tripoli (Lybia) and their geographical distribution in the Mediterranean.- Revista Española de Micropaleontologia, Madrid, vol. 29, nº 3, p. 71-106
D., G. & F. (1998).- Paleoenvironmental bottom water conditions in the early Zanclean of the Capo Rossello area (Agrigento, Sicily).- Bollettino della Società Paleontologica Italiana, Modena, vol. 37, nº 1, p. 61-98.
C. N. (1981).- Guide per il riconoscimento delle specie animali delle acque lagunari e costiere italiane. AQ/1/96. 5. Policheti Serpulidei.- Consiglio Nazionale delle Ricerche, Roma, 187 p.
K., C. & I.K. (2008).- Pliocene-Lower Pleistocene Ostracoda fauna from Insuyu Limestone (Karapinar-Konia/Central Turkey) and its paleoenvironmental implications.- Geological Bulletin of Turkey, Ankara, vol. 51, nº 1, p. 1-31.
R.H. (1984).- Estimating greater paleodepths with ostracodes, especially in past thermospheric oceans.- Palæogeography, Palæoclimatology, Palæoecology, vol. 48, p. 107-141.
G. & N. (1975).- Ostracoda from Lybia.- Pubblicazioni della Stazione Zoologica di Napoli, Napoli, vol. 39, p. 129-135.
G., G. & M. (1975).- Distribution of Ostracoda in the Adriatic Sea.- Pubblicazioni della Stazione Zoologica di Napoli, Napoli, vol. 40 (Suppl.), p. 1-304.
G.S. (1865).- On undescribed fossil Entomostraca from the Brickearth of the Nar.- The Annals and Magazine of Natural History, London, (ser. 3), vol. 1, p. 189-191.
G.S. (1868).- Contributions to the study of the Entomostraca. n. 3. Marine ostracoda from Tenedos.- The Annals and Magazine of Natural History, London, (ser. 4), vol. 2, p. 220-225.
P. (1987).- Les ostracodes indicateurs des milieux et paléomilieux littoraux.- Bulletin de l'Institut de Géologie du Bassin d'Aquitaine, Bordeaux, vol. 41, p. 85-93.
G. & J.-P. (1979).- Les ostracodes des series du Bassin de l'Omo.- Bulletin de l'Institut de Géologie du Bassin d'Aquitaine, Bordeaux, vol. 25, p. 167-199.
M.A., I., D. & M. (1979).- Il Pleistocene in facies limnica di Vittoria (Sicilia meridionale).- Geologica Romana, vol. 18, p. 93-104.
B. (1989).- La malacofauna pleistocenica della Cartiera Molino (Vittoria, Ragusa). In: I., ed., Atti del Terzo Simposio di Ecologia e Paleoecologia delle Comunità Bentoniche.- Tipografia dell'Università, Catania, p. 477-500.
G.C. & R.C. (1996).- Quaternary non-marine ostracods from lake beds in northern Patagonia.- Revista Española de Paleontología, Madrid, vol. 11, p. 143–154.
B., A. & R. (2005).- The Lower-Middle Pleistocene succession of the Coastal Tuscany (Central Italy): new stratigraphic and palaeoecological data based on the ostracod fauna.- Revue de Micropaléontologie, Paris, vol. 48, nº 2, p. 63-81.
A. (1963).- Il Pleistocene in fase levantina di Montallegro (Sicilia Sud-occidentale).- Geologica Romana, vol. 2, p. 59-118.
G. (1965).- Lacustrine Pleistocene in the Lower Liri Valley.- Geologica Romana, vol. 4, p. 291-368.
I. (1985).- La bionomie benthique appliquée à l'étude des peuplements fossiles de la Méditerranée : Contribution des chercheurs italiens.- Tethys, Marseille, vol. 11, nº 3-4, p. 243-248.
I., B., R., G., A., R. (1994).- The Pleistocene "Case Catarinicchia" section (Belice, SW Sicily). In: R., M.G. & J.S., eds., Studies on ecology and paleoecology of benthic communities.- Bollettino della Società Paleontologica Italiana, Modena, spec. vol. 2, p. 93-115.
Y.V. (1962).- Recherches écologiques sur les Bryozoaires Chilostomes en Méditerranée occidentale.- Recueils des Travaux de la Station marine d'Endoume, Marseille, vol. 38, 435 p.
P. & I. (2005).- The use of ostracods from marginal marine brackish waters as bioindicators of modern and Quaternary environmental change.- Palæogeography, Palæoclimatology, Palæoecology, vol. 225, p. 68-92.
E., V., J.-P. & M. (2000).- Environmental variation and foraminiferal test abnormalities.- Environmental Micropaleontology, Topics in Geobiology, vol. 15, p. 191-215.
E. & F. (2008).- Multivariate analysis as a tool to infer the autoecology of extinct ostracods: an example from two Italian late Messinian lago-mare assemblages.- Palæogeography, Palæoclimatology, Palæoecology, vol. 264, p. 288-295.
E. & I. (1998).- Paleoenvironmental analysis of the 250.000 years Quaternary sediment core of Valle di Castiglione (Latium, Italy) using ostracods. In: - S., E. & F., eds., What about Ostracoda!- Bulletin des Centres de Recherche Exploration Production elf-Aquitaine, Pau, vol. 20, p. 70-90.
C. & F. (1989).- Ostracodes et recherche des milieux anciens : Possibilités et limites.- Bulletin de la Société géologique de France, Paris, vol. 8, nº 5, p. 577-588.
C. & M. (1994).- Ostracodes en milieu océanique profond (Atlantique Central) au passage Miocène-Pliocène.- Revue de Micropaléontologie, Paris, vol. 37, nº 4, p. 257-274.
V. (2011).- Holocene ostracods records of the karst lakes sediments, Cres and Mljet islands, Adriatic Sea (Croatia). Joannea Geologie und Paläontologie, Graz, vol. 11, p. 69-70.
J.-G. (1976).- Le sous-ordre des Tubuliporina (Bryozoaires Cyclostomes) en Méditerranée : Écologie et systématique.- Mémoires de l'Institut Océanographique, Monaco, vol. 10, 326 p.
J.-G. (1990).- Deep-water crisiids (Bryozoa: Cyclostomata) from the northeast Atlantic Ocean.- Journal of Natural History, London, vol. 24, p. 1597-1616.
D. van (1986).- Use of ostracodes to recognize downslope contamination in paleobathymetry and a preliminary reappraisal of the paleodepth of Prasas Marls (Pliocene), Crete, Greece.- Geology, vol. 14, p. 856-859.
P.A. (1990).- Freshwater Ostracoda.- Synopses of the British Fauna, London, vol. 42, 228 p.
P.A. (2002).- Freshwater Ostracoda.- CD version, Pisces Conservation Ltd, Hants.
T., M. & M. (2000).- Ostracod (Crustacea) populations as environmental indicators of inter-tropical transitional deltaic environments. An example from the Néra Delta, New Caledonia, South-West Pacific.- Revue de Paléobiologie, Genève, vol. 19, nº 1, p. 207-225.
K.M. (2006).- Climatic characteristics of the late Pleistocene and Holocene continental deposits from southwestern Syria based on palynological data.- Darwiniana, Cordoba, vol. 44, nº 2, p. 329-340.
R.W. & , M.D. (1996).- A rewiew of the stratigraphy of eastern Paratethys (Oligocene-Holocene).- Bulletin of the Natural History Museum, London, vol. 52, nº 1, p. 25-49.
L. (1820).- Histoire des monocles qui se trouvent aux environs de Genève.- J.J. Paschoud, Genève, 258 p.
URL: http://www.biodiversitylibrary.org/item/40489#page/7/mode/1up
F., S., G., I., M., S., M., G. & F. (1984).- Carta geologica della Sicilia Sud Orientale alla scala 1:100.000.- Selca, Firenze.
M., G., C., - P., G., J.-R., O., - F., F., L., O., B. & A.-V. (2006).- Environmental and climatic changes in the Jura mountains (eastern France) during the Late glacial-Holocene transition: a multi-proxy record from Lake Lautrey.- Quaternary Science Reviews, vol. 25, p. 414-445.
S. & R. (2001).- Palaeoceanographical changes recorded by Cenozoic deep sea ostracod assemblages from the South Atlantic and the Southern Ocean (ODP Sites 1087 and 1088).- Lethaia, Oslo, vol. 34, p. 63-83.
I., P., M., F., L., E., M. & E. (1999).- Late Quaternary sea-level changes along the Tyrrhenian coast near Orbetello (Tuscany, central Italy): palaeoenvironmental reconstruction using ostracods.- Marine Micropaleontology, vol. 37, p. 289-311.
K.G. (1963).- A brackish water ostracod fauna from Lago di Patria, near Naples.- Annali dell'Istituto e del Museo di Zoologia dell'Università di Napoli, Napoli, vol. 15 , nº 1, p. 1-13.
C. (1988).- Ostracods récoltés à Paris. Avec une clef pour la determination des espèces européennes du genre Ilyocypris (Crustacea, Ostracoda).- Bulletin de la Société des Naturalistes luxembourgeois, vol. 88, p. 145-163.
C. (2000).- Freshwater ostracoda of western and central Europe. In: J. & P., eds., Süsswasserfauna von Mitteleuropa.- Spektrum Akademischer Verlag, Heidelberg, 522 p.
C. & K. (2003).- Valve surface structure of Candona neglecta , 1887 (Crustacea Ostracoda).- Studia Quaternaria, Warsaw, vol. 21, p. 15-18.
C, A., F., I., W., T., R., R. & R. (2009).- Anatomy of a transgressive systems tract revealed by integrated sedimentological and palaeoecological study: the Barcellona Pozzo di Gotto Basin, northeastern Sicily, Italy. In: G. & W.C., eds., Sedimentary processes, Environments and Basins. A tribute to Peter Friend.- International Association of Sedimentology, Special Publication nº 38, vol. 1, p. 367-400.
S. & J. (2008).- Applications of lacustrine and marginal-marine Ostracoda to palaeoenvironmental reconstruction.- Palæogeography, Palæoclimatology, Palæoecology, vol. 264, p. 211-212.
M.E., N. & G. (1998).- Shelf ostracods distribution in the Italian seas. In: - S., E. & F., eds., What about Ostracoda!- Bulletin des Centres de Recherche Exploration Production elf-Aquitaine, Pau, vol. 20, p. 91-101.
G.W. (1900).- Deutschlands Süsswasser-Ostracoden.- Zoologica, Stuttgart, vol. 30, p. 1-112.
J.W. (1991).- Ecology and palaeoecology of benthic foraminifera.- Longman Scientific and Technical,London, 397 p.
D., R. & - J. (1993).- Los ostracodos y la evolucion paleoambiental del Plioceno inferior de la Cuenca de Tetuan (Marruecos N. Occidental).- Revista Española de Micropaleontologia, Madrid, vol. 25, nº 2, p. 25-61.
A.M. (1862).- X.- Contribution to British carcinology.- The Annals and Magazine of Natural History, London, (ser. 3), vol. 9, p. 43-52.
J.-M. (1982).- Structure and dynamics of assemblages in the benthal. In: O., ed., Marine ecology: a comprehensive, integrated treatise on life in oceans and coastal waters.- Ocean Management, London, vol. 5, nº 1, p. 119-185
J.-M. & J. (1964).- Nouveau manuel de bionomie benthique de la Mer Méditerranée.- Recueil des Travaux de la Station marine d'Endoume, Marseille, vol. 31, nº 47, 137 p.
V., K., - L., F. & G. (2006).- Distribution of recent ostracods in island waters of Sicily (Southern Italy).- Journal of Limnology, Verbania, vol. 65, nº 1, p. 1-8.
F.A. (1808).- XII. Über die Gattung Cypris und drei zu derselben gehörige neue Arten.- Geselschaft Naturforschung Freunde, Berlin, II Jarhgang, p. 83-93 (Tab. III).
- & - (2005).- Pliocene Ilyocyprididae (Ostracoda) from the Ebro Basin (N Spain).- Revue de Micropaléontologie, Paris, vol. 48, p. 37-49
G., K., C., S. & V. (2006).- Small is beautiful: diversity of freshwater ostracods (Crustacea, Ostracoda) in marginal habitats of the province of Parma (Northern Italy).- Journal of Limnology, Verbania, vol. 65, nº 2, p. 121-131.
V., G., M., C., P., A. & K.G. (2003).- Ostracoda of the Italian ricefields thirty years on: new synthesis and hypothesis.- Journal of Limnology, Verbania, vol. 62, nº 1, p. 1-8.
A., R., E. & E. (2013).-Serpuloidean, bryozoan and brachiopod faunas from submarine caves in Sicily.- Bollettino della Società Paleontologica Italiana, vol. 52, nº 3, p. 167-176.
G. (1950).- Gli Ostracodi delle sabbie grigie quaternarie (Milazziano) di Imola (I).- Giornale di Geologia, Bologna, (ser. 2), vol. 21, p. 1-58.
G. (1961).- Frammenti di stratigrafia siciliana.- Rivista mineraria siciliana, Palermo, anno 12, nº 70-72, p. 3-8.
G. (1975).- Contributo alla conoscenza del genere Aurila (Ostracoda, Podocopida).- Bollettino della Società Paleontologica Italiana, Modena, vol. 14, nº 1, p. 27-46.
A.M. & A.B. (2001).- Benthic foraminiferal assemblages and morphological abnormalities as pollution proxies in two Egyptian bays.- Marine Micropaleontology, vol. 41, p. 193–227.
R., A., A., L., A., M. (2013).- Serpula aggregates and their role in deep-sea coral communities in the southern Adriatic Sea.- Facies, Erlangen, vol. 59, p. 663-677.
G.O. (1887).- Nye bitrag ti Kundstkaben om Middelhavets invertebratfauna.- Archiv for Mathematik og Naturvidewskab, Oslo, vol. 12, p. 173-324.
A. (2011).- Ostracods (Crustacea) from floodplain of the Chi River basin, Mahasarakham Province, Thailand, with first record of the male Tanycypris siamesis & , 2009.- Joannea Geologie und Paläontologie, Graz, vol. 11, p. 174.
S., W. & E. (2005).- Ostracods and bivalves from Upper Pleistocene (Tyrrhenian) marine terrace near Altinova (Izmit province, Turkey).- Zitteliana, München, vol. A45, p. 87-114.
F. (2003).- Dati preliminari sulla ostracofauna pliocenica di Capo Milazzo (Sicilia NE).- Bollettino della Società Paleontologica Italiana, Modena, vol. 42, nº 1-2, p. 179-184.
F. (2005).- Ostracodi batiali pleistocenici di Capo Milazzo (Sicilia NE) ed implicazioni paleoambientali.- Rendiconti della Società Paleontologica Italiana, Modena, vol. 2, p. 219-227.
F., A. & S. (2003).- Associazioni ad ostracodi del Pliocene di Centuripe (Sicilia): interpretazione paleoambientale.- Bollettino della Società Paleontologica Italiana, Modena, vol. 42, nº 3, p. 267-280.
W. (1972).- Late Cenozoic Ostracoda in the South Aegean Island Arc.- Utrecht Micropaleontological Bulletins, vol. 6, 187 p. (12 Pls.).
URL: http://dspace.library.uu.nl/handle/1874/205810
A.J. & D.J. (2002).- Ecology of marine, marginal marine and nonmarine Ostracodes. In: J.A. & A.R. (eds.), The Ostracoda: Application in Quaternary Research.- Geophysical Monograph, Washington, vol. 131, p. 37-64.
M.V., T., O. & D.M. (2005).- Foraminiferal and ostracod ecological patterns in coastal environments of SE Andros Island (Middle Aegean Sea, Greece).- Revue de Micropaléontologie, Paris, vol. 48, p. 279-302.
S., O., D. & E. (2014).- Distribution, diversity and ecological characteristic of freshwater ostracoda (Crustacea) in shallow aquatic bodies of the Ankara Region Turkey.- Wetlands, vol. 34, p. 309-324.
C.W. (1957).- Sur les ostracodes du Quaternaire récent de Pays-Bas et leur utilisation dans l'étude géologique des dépôts holocènes.- Mouton & co. 'c-Gravenhage, 259 p.
V., M. & M. (1998).- Morphological deformities of benthic foraminiferal tests in response to pollution by heavy metals: implications for pollution monitoring.- Journal of Foraminiferal Research, Lawrence, vol. 28, p. 177–200.
M. (1986).- Fauna dels bryozous dels Països Catalans.- Institut d'Estudis Catalans, Seccion de Ciències, Barcelona, vol. 84, 833 p.
C., F., P. & M. (2011).- Distribution des ostracods dans les sédiments de subsurface de la Sebkha el-Guettiate (Skhira, Golge de Gabès). Intérêt pour la reconstitution des paléo-environnements de l'Holocène.- Carnets Géol., Madrid, CG2011_A03, p. 63-81.
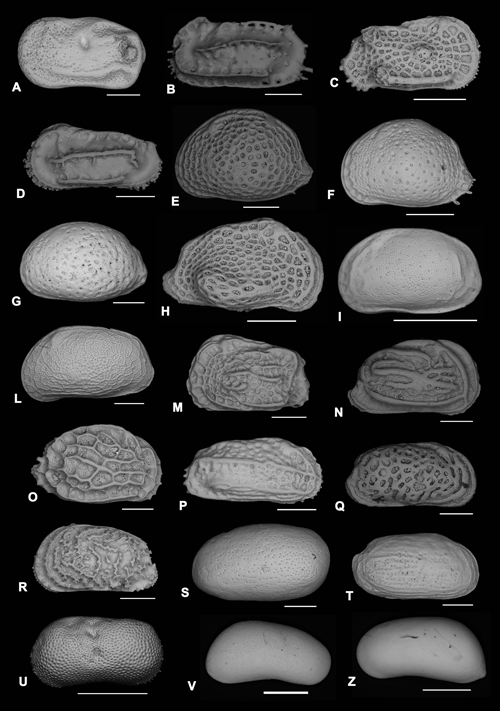
Click on thumbnail to enlarge the image.
Plate 1: Ostracoda
A) Cytherelloidea beckmanni -, 1971. LV external lateral view. Scale bar 200 µm;
B) Carinocythereis whitei (, 1850). LV external lateral view. Scale bar 250 µm;
C) Hermanites haidingeri (, 1850). RV external lateral view. Scale bar 350 µm;
D) Costa batei (, 1866). LV external lateral view. Scale bar 250 µm;
E) Aurila gr. punctata (, 1830). LV external lateral view. Scale bar 200 mm;
F) Aurila gr. convexa (, 1850). LV external lateral view. Scale bar 300 µm;
G) Aurila sp. 1. LV external lateral view. Scale bar 250 µm;
H) Aurila balanoides , 1983. RV external lateral view. Scale bar 200 µm;
I) Aurila sp. 2. LV external lateral view. Scale bar 500 µm;
L) Aurila arborescens (, 1865). LV external lateral view. Scale bar 200 µm;
M) Quadracythere prava (, 1850). LV external lateral view. Scale bar 250 µm;
N) Graptocythere hscripta (, 1900). RV external lateral view. Scale bar 200 µm;
O) Mutilus cf. laticancellatus (, 1928). RV external lateral view. Scale bar 200 µm;
P) Cistacythereis rubra (, 1894). RV external lateral view. Scale bar 250 µm;
Q) Urocythereis cf. sororcula (, 1880). RV external lateral view. Scale bar 250 mm;
R) Urocythereis sp. 1. LV external lateral view. Scale bar 200 µm;
S) Cytheretta subradiosa (, 1836). LV external lateral view. Scale bar 200 mm;
T) Cytheretta adriatica , 1952. RV external lateral view. Scale bar 500 µm;
U) Ilyocypris gibba , 1808. LV external lateral view. Scale bar 500 µm;
V) Candona angulata , 1900. RV (female) external lateral view. Scale bar 500 µm;
Z) Candona angulata , 1900. LV (female) external lateral view. Scale bar 500 µm.
Table 1: List of the ostracods found in the Cartiera Molino section (X = rare, XX = abundant, XXX= very abundant).
| SPECIES | SAMPLES | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Candona angulata , 1900 | x | ||||||||
| Candona neglecta (, 1887) | x | x | |||||||
| Cypridopsis vidua (O.F. , 1776) | x | ||||||||
| Eucypris virens (, 1820) | x | ||||||||
| Herpetocypris sp. | x | x | |||||||
| Ilyocypris monstrifica (, 1862) | x | x | |||||||
| Ilyocyris gibba (, 1808) | x | ||||||||
| Loxoconcha tumida , 1902 | x | ||||||||
| Xestoleberis dispar , 1894 | x | ||||||||
| Aurila sp. | x | ||||||||
| Aurila arborescens (, 1865) | x | ||||||||
| Aurila cf. cruciata (, 1950) | xx | xxxx | |||||||
| Urocythereis sororcula (, 1880) | xxx | xxx | x | ||||||
| Urocythereis sp.1 | x | x | |||||||
| Loxoconcha gibberosa , 1878 | xxx | ||||||||
| Loxoconcha bairdi , 1894 | x | ||||||||
| Loxoconcha stellifera , 1894 | x | ||||||||
| Carinocythereis whitei (, 1850) | xx | ||||||||
| Cytheretta subradiosa (, 1838) | x | ||||||||
| Cytheretta adriatica , 1952 | x | x | |||||||
| Mutilus cf. laticancellatus (, 1928) | x | x | |||||||
| Aurila gr. convexa (, 1850) | xx | xxx | xxx | xxxx | |||||
| Costa batei (, 1866) | x | xxx | xxx | xxx | |||||
| Graptocythere hscripta (, 1900) | x | xx | xxx | x | |||||
| Neonesidea mediterranea (, 1894) | x | xx | xxx | ||||||
| Urocythereis margaritifera (, 1894) | x | xx | x | ||||||
| Semicytherura paradoxa (, 1894) | x | x | x | ||||||
| Paracytheridea gr. depressa , 1894 | x | x | x | ||||||
| Semicytherura sp. 1 | x | x | x | ||||||
| Sagmatocythere napoliana (, 1963) | x | x | |||||||
| Callistocythere lobiancoi (, 1894) | x | x | |||||||
| Cytherelloidea beckmanni , 1971 | x | x | |||||||
| Cytherella alvearium , & , 1976 | x | x | |||||||
| Hemicytherura defiorei , 1953 | x | ||||||||
| Aurila balanoides 1983 | x | ||||||||
| Aurila prasina , 1971 | x | ||||||||
| Loxoconcha rhomboidea (, 1855) | x | ||||||||
| Caudites calceolatus (, 1853) | x | ||||||||
| Leptocythere lagunae , 1958 | x | ||||||||
| Tetracytherura angulosa (, 1880) | x | ||||||||
| Grinioneis haidingeri (, 1850) | x | ||||||||
| Kangarina abyssicola (, 1894) | x | ||||||||
| total number of species | 23 | 19 | 14 | 6 | 0 | 0 | 7 | 3 | |
Table 2: List of the foraminifers found in the Cartiera Molino section (X = rare, XX = abundant, XXX= very abundant).
| SPECIES | SAMPLES | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Benthos | |||||||||
| Ammonia beccarii (, 1758) | x | x | x | x | x | ||||
| Ammonia tepida (, 1926) | x | ||||||||
| Asterigerinata mamilla (, 1858) | x | x | x | ||||||
| Bolivina alata (, 1862) | x | ||||||||
| Bulimina marginata (, 1826) | x | ||||||||
| Cancris auriculus ( & , 1798) | x | ||||||||
| Cancris sp. | x | ||||||||
| Cibicides lobatulus ( & , 1798) | x | x | x | x | |||||
| Cribroelphidium sp. | x | ||||||||
| Elphidium aculeatum (, 1846) | x | x | x | ||||||
| Elphidium complanatum (, 1839) | x | x | |||||||
| Elphidium crispum (, 1758) | x | x | x | x | |||||
| Elphidium sp. | x | ||||||||
| Paracassidulina neocarinata (, 1950) | x | ||||||||
| Rosalina globularis , 1826 | x | x | |||||||
| total number of species | 7 | 8 | 4 | 6 | 6 | ||||
| Plankton | |||||||||
| Globigerina calida , 1962 | x | x | |||||||
| Globigerinoides elongatus (, 1839) | x | ||||||||
| Globigerinoides ruber (, 1839) | x | x | x | x | |||||
| Globigerinoides trilobus (, 1850) | x | ||||||||
| Globorotalia inflata (, 1839) | x | x | x | x | |||||
| Globorotalia margaritae & , 1965 (R) | x | x | |||||||
| Globorotalia puncticulata , 1832 (R) | x | x | |||||||
| Neogloboquadrina pachyderma (, 1861) | x | ||||||||
| Orbulina suturalis (, 1951) | x | ||||||||
| Orbulina universa , 1839 | x | ||||||||
| total number of species | 2 | 3 | 7 | 4 | 1 | 2 | |||
| (R)= reworked | |||||||||
Table 3: List of the serpuloideans found in the Cartiera Molino section (X = rare, XX = abundant, XXX= very abundant).
| SPECIES | SAMPLES | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Serpula vermicularis , 1767 | x | ||||||||
| Hydroides dianthus (, 1873) | x | ||||||||
| Hydroides elegans (, 1883) | x | ||||||||
| Hydroides sp. | xxx | ||||||||
| Vermiliopsis labiata (O.G. , 1861) | x | ||||||||
| Vermiliopsis striaticeps (, 1862) | x | xxx | x | ||||||
| Spirobranchus polytrema (, 1844) | x | ||||||||
| Janua pagenstecheri (, 1866) | x | ||||||||
| Neodexiospira pseudocorrugata (, 1905) | x | ||||||||
| total number of species | 1 | 9 | 1 | ||||||
Table 4: List of the bryozoans found in the Cartiera Molino section (X = rare, XX = abundant, XXX= very abundant).
| SPECIES | SAMPLES | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Platonea stoechas , 1976 | x | xxx | x | ||||||
| ? Tubulipora liliacea (, 1766) | x | ||||||||
| ? Tubulipora plumosa , 1898 | x | x | |||||||
| ? Annectocyma sp. | xx | xx | x | x | |||||
| Crisia fistulosa (, 1867) | xxx | xxx | x | ||||||
| ? Crisia pyrula , 1990 | xxx | xx | |||||||
| Crisia spp. | xxx | xx | x | ||||||
| Calpensia nobilis (, 1796) | x | x | |||||||
| Scrupocellaria sp. | x | x | |||||||
| ? Watersipora sp. | x | ||||||||
| Schizomavella sp. | x | x | |||||||
| total number of species | 8 | 9 | 4 | 1 | |||||