◄ Carnets Geol. 16 (3) ►
Contents
[Introduction]
[Material and methods]
[Results]
[Conclusions]
and ... [Bibliographic references]
Corresponding author
Department of Environmental Sciences and Earth Sciences, University of Milano Bicocca, Piazza della Scienza 4, 20126
Milano (Italy)
Department of Geological sciences, Masaryk University, Kotlářská 2, 611 37 Brno (Czech Republic)
Department of Environmental Sciences and Earth Sciences, University of Milano Bicocca, Piazza della Scienza 4, 20126
Milano (Italy)
Published online in final form (pdf) on February 20, 2016
[Editor: Bruno ;
language editor: Stephen ]
The examination of fossil Lithothamnion specimens ranging in age from the Early Miocene to the Pleistocene revealed the occurrence of multiporate conceptacle chambers pitted with depressions. This is the diagnostic feature of Lithothamnion crispatum, a cosmopolitan hapalidiacean with a wide depth range in modern oceans. The comparison of the microscopic anatomy of both the fossil and modern L. crispatum confirmed that they are conspecific. Therefore, this species has a long stratigraphic distribution starting at least 20 My ago, without significant morphological changes in either reproductive or vegetative anatomy.
Morphology; multiporate conceptacle roof; Tethys; Melobesioideae; Miocene.
G., J. & D. (2016).- Lithothamnion crispatum: long-lasting species of non-geniculate coralline algae (Rhodophyta, Hapalidiales).- Carnets Geol., Madrid, vol. 16, nº 3, p. 27-41.
Lithothamnion crispatum : une espčce d'algue coralline non-geniculée persistante dans le temps (Rhodophyta, Hapalidiales).- La révision d'exemplaires fossiles de Lithothamnion dont l'âge varie du Miocčne inférieur au Pleistocčne a montré la présence de chambres de conceptacles multiporés marquées par des dépressions, ce qui constitue le caractére diagnostique de Lithothamnion crispatum, une algue de la Famille des Hapalidiaceae, cosmopolite et vivant ŕ une large gamme de profondeur dans les océans actuels. La comparaison des structures microscopiques de spécimens fossiles et modernes de L. crispatum confirme qu'ils doivent ętre considérés comme conspécifiques. Par conséquent, cette espčce a une répartition stratigraphique trčs grande, commençant il y a au moins 20 millions d'années, sans variation morphologique significative dans les parties reproductives ou végétatives de son anatomie.
Morphologie ; conceptacles ŕ toit multiporé ; Téthys ; Melobesioideae ; Miocčne.
According to taxonomic uniformitarianism it is possible to extrapolate the ecological significance of modern species to their fossil counterparts. In most cases this core principle of paleoecology can be safely applied at least for paleoenvironments not older than the Miocene ( et al., 1997; et al., 2000). To accomplish this goal, recent species need reliable identification in the fossil record achievable through the application of the same criteria used in biological classification. In this way, the biological and paleontological classifications can be made consistent as far as fossil preservation allows. For coralline algae this approach has been developed in various contributions ( et al., 1993; et al., 1996, 1997; and , 1999, 2000; et al., 2009; et al., 2009; et al., 2012; et al., 2014; et al., 2015).
The aim of this paper is to contribute to this development by introducing a new diagnostic character never seen nor described before in the fossil material: the presence of pits surrounding the pore canals in the roof of the sporangial conceptacles. This is the key morphological feature of the non-geniculate coralline Lithothamnion crispatum , 1878, unknown as a fossil until now. Fossils from the Pleistocene and the Miocene have been compared with modern Mediterranean specimens of L. crispatum to assess and study their relationship. The modern cosmopolitan distribution of L. crispatum includes the Mediterranean ( and , 2003; et al., 2011), the Atlantic Ocean ( et al., 2000; et al., 2010), the Indian Ocean ( et al., 1996), the Pacific coast of Australia ( et al., 2003) and the northern coast of New Zealand ( et al., 2015). Living specimens have been collected from 2 to 80 m of water depth, as rhodoliths or crusts attached on hard substrates, particularly in well lit waters ( et al., 2000; et al., 2010; et al., 2011; et al., 2013; et al., 2015).
This species has a complex taxonomic history (details in et al., 2011). In 2000 et al. established that Lithothamnion superpositum is conspecific with Lithothamnion indicum. Subsequently et al. (2010) merged L. superpositum with Lithothamnion heteromorphum. Finally, in 2011, et al. synonymized L. heteromorphum with L. crispatum, the Mediterranean species with nomenclatural priority. As a consequence of these taxonomic revisions, the geographic and ecological distribution of the species has become very broad, encompassing all the oceans and both hemispheres. The key feature that allowed synonymizing these species is the occurrence of pits over the roof of the multiporate conceptacles, a unique feature among Hapalidiales and a diagnostic feature in the delimitation of this Lithothamnion species ( and , 1995). These roof pits are the consequence of the disintegration of the outermost cells in filaments surrounding the pore canals. In the resulting structure the pore canal is slightly raised in the center of a rosette of degenerate cells.
The specimens analyzed come from different localities, with some being studied in previous investigations (Table 1). Different specimens from the same sample or from the same outcrop were grouped together for analysis. Morphological data on Recent L. crispatum come from samples collected in the Egads Archipelago of the Western Mediterranean (Marettimo Island, et al., 2011).
Fossil samples were cut with an abrasive rock saw, and the porosity was then filled with epoxy resin. The surfaces were polished with silicon carbide and glued onto standard glass slides. The excess material was subsequently cut off and the thickness of the sample was reduced to less than 30 μm. The thin sections of coralline algal samples were then observed under light microscope to study and measure their anatomical features. Growth-forms terminology follows et al. (1993). Vegetative anatomy was measured along longitudinal radial sections ( et al., 2007; et al., 2008). The diameter of the cells was measured including the cell wall; cell length was measured as the distance between two primary pit connections including the cell wall ( et al., 1996). Conceptacles were measured along their axial section, i.e., the longitudinal section that cuts a conceptacle medially so that the pore canal is completely visible ( et al., 1984), in accordance to et al. (2007) and et al. (2008). Very large conceptacle chambers, clearly resulting from the merging of two nearby conceptacles, were not considered in statistical analyses. The diameter of the roof pits was measured either in the axial section of conceptacles or in the equatorial section tangential to the roof-top layer of cells. The length of the degenerate cells (i.e., the depth of the roof pit around the pore canal) was measured in the axial section of the conceptacles.
Analysis of variance (ANOVA) was employed to support the morphological comparison of the specimens and 's test was used to assess the equality of variances. Since ANOVA can analyze only one character at time, the H/D ratio of asexual conceptacle chambers was used, because it summarizes both conceptacle dimensions. In order to analyze differences and similarities between the samples further, multivariate statistics were performed with PRIMER 6 (, 1977; et al., 1982; and , 2006). - (B-C) samples similarity was calculated on average standardized values of the ventral core (hypothallium) and peripheral zone (perithallium) cell size, conceptacles diameter (D) and height (H), H/D ratio and roof thickness. The size of roof pits was not included in this analysis because of the small number of observations in the fossil and because no reference values was available for the modern specimens. Results, based on B-C similarity, were plotted as hierarchical agglomerative dendrograms.
Table 1: Fossil samples analyzed.
| Geographic Position | Age | Nº of thin sections | Collector | Useful references | |
| NW Italy-Tertiary Piedmont Basin-Villa San Bartolomeo | Burdigalian | 1 | G. | et al., 1996, and et al., 2015, for further details regarding the outcrop, the geological setting and coralline algae flora | |
| NW Italy-Tertiary Piedmont Basin-Uviglie | Burdigalian | 3 | GC | ||
| NW Italy-Tertiary Piedmont Basin-Torre Veglio | Burdigalian | 2 | G. | ||
| S France-Sommičres Basin-Souvignragues | Burdigalian | 1 | GC | and , 2012, for further details on the geological setting of the basin | |
| Czech Republic-Central Parathethys-Židlochovice | upper part of Lower Langhian | 1 | JH | and , 1997; et al., 2005; et al., 2007; et al., 2008; et al., 2015, for information regarding the geological setting and coralline algae flora | |
| Romania-Central Parathethys-Lopadea Veche | upper part of Lower Langhian | 1 | JH | ||
| Slovakia-Parathethys-Stupava-Vrchná Hora | Upper Langhian | 1 | JH | ||
| Slovakia-Parathethys-Sandberg | Lower Serravallian | 1 | JH | ||
| S Italy-Sicily-Castelluccio | Pleistocene | 3 | DB |
Table 2: Comparison of features and biometry of modern and fossil L. crispatum. The specimens of L. crispatum from the Lower Serravallian of Slovakia, Sandberg locality, were poorly preserved. Since it was not possible to make an appropriate number of measurements for most of the parameters, they are omitted from the table; VC= ventral core; PF=peripheral zone; s.d. =standard deviation; //= insufficient data cover.
| L. crispatum Burdigalian Tertiary Piedmont Basin NW Italy |
L. crispatum Burdigalian Sommičres Basin S France |
L. crispatum upper part of Lower Langhian Židlochovice Czech Republic |
L. crispatum upper part of Lower Langhian Lopadea Veche Romania |
L. crispatum Upper Langhian Stupava Slovakia |
L. crispatum Pleistocene Castelluccio S Italy |
L. crispatum Recent Marettimo S Mediterranean |
|
| Thallus organization | monomerous | monomerous | monomerous | monomerous | monomerous | monomerous | monomerous |
| Ventral core structure | non coaxial plumose |
non coaxial plumose |
non coaxial plumose |
non coaxial plumose |
non coaxial plumose |
non coaxial plumose |
non coaxial plumose |
| VC cells length | 11-28 μm [16 mean; 3.2 s.d.; n=80] |
// | 10-28 μm [16.8 mean; 4.5 s.d.; n=30] |
9-26 μm [16.7 mean; 3.6 s.d.; n=32] |
10-26 μm [16.9 mean; 3.5 s.d.; n=42] |
13-25 μm [18 mean; n=10] |
11-34 μm [18.6 mean; 4.2 s.d.; n=258] |
| VC cells height | 7-12 μm [9.6 mean; 1.6 s.d.; n=80] |
// | 7-12 μm [10.5 mean; 1.5 s.d.; n=30 |
7-11 μm [9.6 mean; 1.2 s.d.; n=32] |
6-11 μm [9 mean; 1.5 s.d.; n=42] |
7-11 μm [8.5 mean; n=10] |
5.5-15 μm [9.5 mean; 2.2 s.d.; n=258] |
| PF zonation | yes | yes | yes | yes | yes | yes | yes |
| PF cells lenght | 8-22 μm [13.3 mean; 2.3 s.d.; n=160] |
10-15 μm [12.5 mean; 1.5 s.d.; n=30] |
9-20 μm [13.7 mean; 3 s.d.; n=50] |
8-31 μm [14.3 mean; 4.5 s.d.; n=50] |
11-20 μm [13.8 mean; 2.4 s.d.; n=42] |
9-19 μm [13.2 mean; 2.5 s.d.; n=90] |
7-25 μm [15 mean; 3.1 s.d.; n=332] |
| PF cells diameter | 8-14 μm [10.2 mean; 0.9 s.d.; n=160] |
9-12 μm [10.5 mean; 1.5 s.d.; n=30] |
9-13 μm [10.5 mean; 0.9 s.d.; n=50] |
9-15 μm [11.2 mean; 1.2 s.d.; n=50] |
9-12 μm [10.4 mean; 0.9 s.d.; n=42] |
8-13 μm [10.4 mean; 1 s.d.; n=90] |
7-16 μm [12 mean; 1.7 s.d.; n=332] |
| Conceptacle chamber diameter |
265-425 μm [340 mean; 55 s.d.; n=30] |
// | 280-510 μm [355 mean; 60 s.d.; n=15] |
265-415 μm [350 mean; 50 s.d.; n=14] |
260-495 μm [390 mean; 80 s.d.; n=14] |
290-550 μm [425 mean; 70 s.d.; n=22] |
270-720 μm [430 mean; 128 s.d.; n=18] |
| Conceptacle chamber height |
90-135 μm [115 mean; 10 s.d.; n=30] |
// | 95-140 μm [115 mean; 15 s.d.; n=15] |
110-145 μm [130 mean; 10 s.d.; n=14] |
110-150 μm [125 mean; 15 s.d.; n=14] |
105-190 μm [140 mean; 20 s.d.; n=22] |
100-315 μm [177 mean; 66 s.d.; n=18] |
| Conceptacle H/D ratio |
0.29-0.47 μm [0.34 mean; 0.05 s.d.; n=30] |
// | 0.25-0.49 μm [0.33 mean; 0.07 s.d.; n=15] |
0.29-0.5 μm [0.38 mean; 0.06 s.d.; n=14] |
0.27-0.44 μm [0.33 mean; 0.06 s.d.; n=14] |
0.26-0.39 μm [0.33 mean; 0.05 s.d.; n=22] |
0.15-0.57 μm [0.41 mean; 0.11 s.d.; n=19] |
| Roof thickness | 27-48 μm [36 mean; 6 s.d.; n=30] |
// | 36-50 μm [42 mean; 5 s.d.; n=15] |
40-62 μm [50 mean; 6 s.d.; n= 14] |
34-51 μm [40 mean; 5 s.d.; n=14] |
38-54 μm [46 mean; 6 s.d.; n=22] |
20-45 μm [30 mean] |
| Number of roof cells | 3 to 4 | // | 3 to 4 | 3 to 6 | 3 to 4 | 3 to 5 | 4 to 6 |
| Diameter of the pits | 19-36 μm [28 mean; 5 s.d.; n=20] |
// | 26-47 μm [31 mean; 5 s.d.; n=17] |
43-59 μm [49 mean; 6 s.d.; n=18] |
26-44 μm [32 mean; 4 s.d.; n=20] |
26-38 μm [34 mean; 3.5 s.d.; n=20] |
// |
| Lenght of the degenerate cells | 9-20 μm [14 mean; 4 s.d.; n=20] |
// | 15-26 μm [20 mean; 3 s.d.; n=17] |
20-38 μm [29 mean; 5 s.d.; n=18] |
12-24 μm [18.5 mean; 3 s.d.; n=20] |
15-24 μm [19.3 mean; 2.5 s.d.; n=20] |
// |
| Number of rosette cells | 6 to 7 | // | // | 6 to 7 | // | // | 5 to 7 |
| Conceptacles position | slightly raised | slightly raised | slightly raised | slightly raised | slightly raised | slightly raised | slightly raised |
Class Florideophyceae , 1960
Subclass Corallinophycidae & , 2007
Order Hapalidiales et al., 2015
Family Hapalidiaceae (, 1864) et al., 2003
Subfamily Melobesioideae , 1885
Genus Lithothamnion , 1897
Lithothamnion crispatum , 1878
For the complete list of heterotypic synonyms refer to et al. (2011).
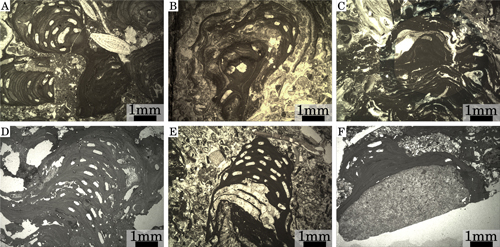
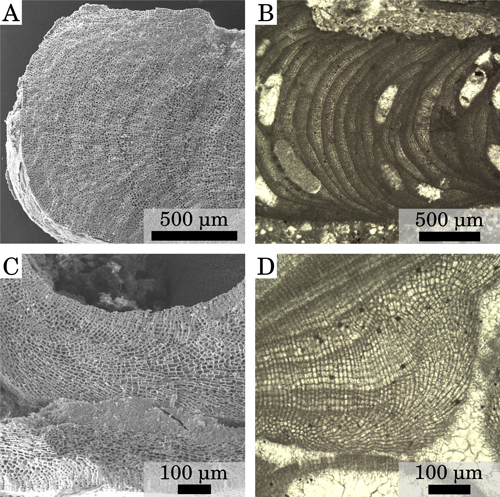
Fruticose to foliose growth form (Fig. 1 ![]() ). Protuberances from cylindrical to lamellate, up to 6 mm long and 3 mm wide and commonly club-shaped. Thalli with monomerous organization, with crustose portions 100 to 500 μm thick (Fig.
2.A-B
). Protuberances from cylindrical to lamellate, up to 6 mm long and 3 mm wide and commonly club-shaped. Thalli with monomerous organization, with crustose portions 100 to 500 μm thick (Fig.
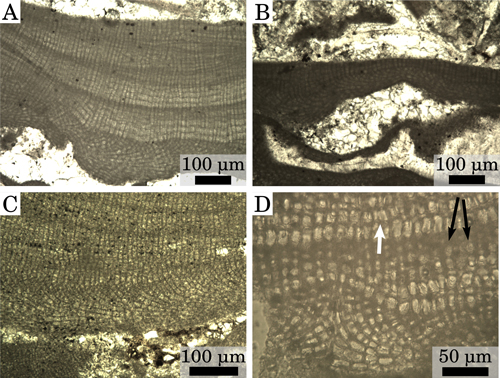
2.A-B ![]() ). Ventral core up to 100 μm thick composed of plumose filaments that arch toward the thallus surface to form the peripheral zone (Fig.
2.C
). Ventral core up to 100 μm thick composed of plumose filaments that arch toward the thallus surface to form the peripheral zone (Fig.
2.C ![]() ). Ventral core cells are rectangular, 9 to 28 μm in length and 6 to 12 μm in diameter
(Table 2). Peripheral zone with alternation of larger cells and smaller cells (Fig.
2.A
). Ventral core cells are rectangular, 9 to 28 μm in length and 6 to 12 μm in diameter
(Table 2). Peripheral zone with alternation of larger cells and smaller cells (Fig.
2.A ![]() ). Peripheral zone cells rectangular, 8 to 31 μm in length and 8 to 15 μm in diameter
(Table 2). Primary pit connections connect cells of the same filament while cells of adjacent filaments are connected by cells fusions (Fig.
2.D
). Peripheral zone cells rectangular, 8 to 31 μm in length and 8 to 15 μm in diameter
(Table 2). Primary pit connections connect cells of the same filament while cells of adjacent filaments are connected by cells fusions (Fig.
2.D ![]() ); in
Recent specimens secondary pits connections have not been observed (see et al.,
2011). Trichocytes have not been observed. It was not possible to observe clearly the shape of epithallial cells either due to the abrasion of the upper layer of cells or the excessive thickness of the sections. No significant difference in length was observed between the alleged sub-epithallial initials and their inward derivatives, in agreement with the observations
of Recent specimens of the species (
et al., 2010,
Fig. 4; et al., 2011, Figs. 6 & 10-11).
); in
Recent specimens secondary pits connections have not been observed (see et al.,
2011). Trichocytes have not been observed. It was not possible to observe clearly the shape of epithallial cells either due to the abrasion of the upper layer of cells or the excessive thickness of the sections. No significant difference in length was observed between the alleged sub-epithallial initials and their inward derivatives, in agreement with the observations
of Recent specimens of the species (
et al., 2010,
Fig. 4; et al., 2011, Figs. 6 & 10-11).
Click on thumbnail to enlarge the image.
Figure 1: Growth-forms of fossil specimens. (A) Branches, Upper Langhian, Slovakia, Stupava-Vrchná Hora. (B) Wavy crusts, upper part of Lower Langhian, Czech Republic, Židlochovice. (C) Crusts in a rhodolith, Burdigalian, NW Italy, Tertiary Piedmont Basin, Villa San Bartolomeo. (D) Branch formed by stacked crusts, Pleistocene, S Italy, Castelluccio. (E) Branch formed by stacked crusts, upper part of Lower Langhian, Romania, Lopadea Veche. (F) Epilithic crust, Pleistocene, S. Italy, Castelluccio.
Click on thumbnail to enlarge the image.
Figure 2: Vegetative anatomy. (A) Thick crust, upper part of Lower Langhian, Romania, Lopadea Veche. (B) Thin crust, upper part of Lower Langhian, Romania, Lopadea Veche. (C) Plumose ventral core, Burdigalian, NW Italy, Tertiary Piedmont Basin, Torre Veglio. (D) High-magnification detail of a secondary ventral-core and of the overlying peripheral zone, Burdigalian, NW Italy, Tertiary Piedmont Basin, Uviglie; white arrow=cell fusion; black arrows=cell filaments with visible primary pits.
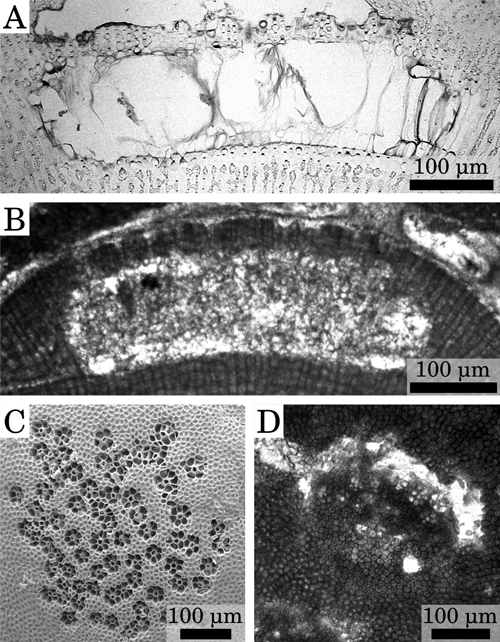
Only multiporate conceptacles have been observed in the
specimens studied. Conceptacle chambers are generally sub-rectangular in shape, 260 to 550 μm in diameter and 90 to 190 μm in height
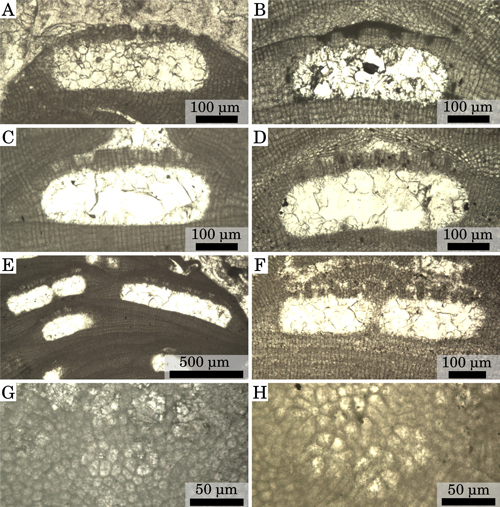
(Table 2; Fig. 3.A-D ![]() ). Conceptacles with very large diameter (generally 700 μm but up to 1000 μm) may result from the merging of two nearby reproductive chambers since
such large structures were always observed close to adjacent couples of normal-sized conceptacles with the separating wall partially dissolved (Fig.
3.E-F
). Conceptacles with very large diameter (generally 700 μm but up to 1000 μm) may result from the merging of two nearby reproductive chambers since
such large structures were always observed close to adjacent couples of normal-sized conceptacles with the separating wall partially dissolved (Fig.
3.E-F ![]() ). The conceptacles protrude slightly above the surrounding thallus surface (Fig.
3.A-F
). The conceptacles protrude slightly above the surrounding thallus surface (Fig.
3.A-F ![]() ). The H/D (height/diameter) ranges between 0.25 and 0.5
(Table 2). The roof of the conceptacle is 27 to 62 μm in thickness and is composed of 3 to 6 cells
(Table 2). The roof of the conceptacles is pitted with depressions, resulting from the disintegration of the uppermost cells of the filaments around the pore canals. The roof pits have a diameter ranging from 19 to 59 μm
(Table 2; Fig. 3
). The H/D (height/diameter) ranges between 0.25 and 0.5
(Table 2). The roof of the conceptacle is 27 to 62 μm in thickness and is composed of 3 to 6 cells
(Table 2). The roof of the conceptacles is pitted with depressions, resulting from the disintegration of the uppermost cells of the filaments around the pore canals. The roof pits have a diameter ranging from 19 to 59 μm
(Table 2; Fig. 3 ![]() ). The
degenerate cells are 9 to 38 μm in length
(Table 2). Rosettes of degenerate cells around the pore canal were observed
in sections tangential to the roof of the conceptacles, with 6 to 7 cells
counted around the pore canal
(Table 2; Fig. 3.G-H
). The
degenerate cells are 9 to 38 μm in length
(Table 2). Rosettes of degenerate cells around the pore canal were observed
in sections tangential to the roof of the conceptacles, with 6 to 7 cells
counted around the pore canal
(Table 2; Fig. 3.G-H ![]() ).
).
Click on thumbnail to enlarge the image.
Figure 3: Reproductive anatomy. (A) Multiporate conceptacle, Burdigalian, NW Italy, Tertiary Piedmont Basin, Villa San Bartolomeo. (B) Multiporate conceptacle, Pleistocene, S. Italy, Castelluccio. (C) Multiporate conceptacle, upper part of Lower Langhian, Romania, Lopadea Veche. (D) Multiporate conceptacle, Upper Langhian, Slovakia, Stupava- Vrchná Hora. (E) Conceptacles almost merged (left) and conceptacles fully merged (right), upper part of Lower Langhian, Romania, Lopadea Veche. (F) Conceptacles almost merged, upper part of Lower Langhian, Czech Republic, Židlochovice. (G) Rosette of degenerate cells around the pore canals, Pleistocene, S. Italy, Castelluccio. (H) Rosette of degenerate cells around the pore canals, upper part of Lower Langhian, Romania, Lopadea Veche.
's test verified that the variances of the H/D ratio of the samples are homogeneous, allowing the use of ANOVA (F distribution with 8 and 97 degrees of freedom). The calculated F-ratio is 1.64,
so that we cannot reject the null hypothesis that the samples belong to a single population.
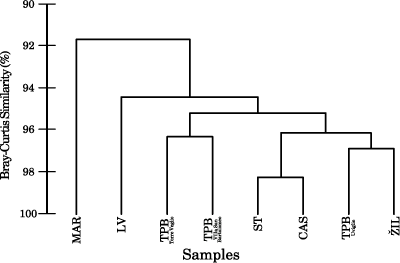
That is, ANOVA demonstrates that there are no statistically significant differences between the mean H/D ratios of the conceptacles of the different samples. Multivariate statistical analysis of the main biometric variables of vegetative and reproductive anatomy shows that all the samples are grouped together at a very high level of B-C similarity (>90%). The dendrogram displays two main clusters:
fossil and modern L. crispatum (Fig. 4 ![]() ). Within the fossils, the largest difference is recognized between the Romanian sample of Lopadea Veche and the remaining fossil samples (Fig. 4
). Within the fossils, the largest difference is recognized between the Romanian sample of Lopadea Veche and the remaining fossil samples (Fig. 4 ![]() ). This latter group is characterized by a >95% of B-C similarity and encompasses samples of different age and different geographical origin (Fig. 4
). This latter group is characterized by a >95% of B-C similarity and encompasses samples of different age and different geographical origin (Fig. 4 ![]() ).
).
Click on thumbnail to enlarge the image.
Figure 4: Multivariate statistical analysis based on B-C similarity of the average value for each specimen of vegetative cell size and morphological characters of the multiporate conceptacles (see text for details); hierarchical agglomerative dendrogram. TPB=Tertiary Piedmont Basin, Burdigalian. ŽIL= Židlochovice, upper part of Lower Langhian, Czech Republic. LV=Lopadea Veche, upper part of Lower Langhian, Romania. ST=Stupava- Vrchná Hora, Upper Langhian, Slovakia. CAS=Castelluccio, Pleistocene, S. Italy. MAR=Marettimo, modern, Mediterranean.
The fossils occur in various depositional environments and are associated with different benthic organisms.
In the outcrops of Tertiary Piedmont Basin, coralline algae dominate the skeletal assemblages, with barnacles and larger benthic foraminifers also very common. These rhodalgal carbonates were deposited in a mesotrophic, high-energy environment at tropical latitudes ( et al., 2000; et al., 2005; et al., 2015).
The Sommičres Basin was a small inlet of the Alpine Molasse Basin and located at the same latitude as the Tertiary Piedmont basin ( et al., 2000). Bryozoans, barnacles, echinoids and coralline algae were the main carbonate producers; larger benthic foraminifers were, in this case, very rare. These heterozoan carbonates were deposited in a tide-controlled environment under mesotrophic conditions as suggested by bryozoan dominance and the presence of authigenic phosphates ( and , 2012).
Red algal limestones from Židlochovice were deposited in a stable shallow normal marine environment with low terrigenous input and seagrass meadows ( et al., 2008, 2014). The sediments were deposited in a warm temperate to subtropical climate with marked seasonality ( et al., 2014). Coralline algal to coralline algal-bryozoan limestones point to a cooler environment than the coral – coralline algal limestones of the southern sites of the Central Paratethys ( et al., 2005; et al., 2008).
Limestones from Lopadea Veche correspond to the Gârbova de Sus Formation (Transylvanian Basin) that is characterized by nodular limestones ( and , 1997). The foraminiferal associations suggest that limestones and marls were deposited in a shallow water environment. Other common components are bivalves, bryozoans and rhodoliths ( and , 1997). The occurrence of patch reefs in the distinct reefal Podeni Member (limestone of Gârbova de Sus Formation) points to subtropical condition ( et al., 2007).
Sands, sandstones and limestones of the Stupava Member from the Vienna Basin contains the large benthic foraminifer Amphistegina ( et , 2013) in high abundance. Sandy limestones were deposited within a dynamic environment of normal salinity at the proposed depth of 35-80 m, affected by terrigenous input. The studied site was located in a warm, temperate to subtropical climatic zone ( and , 2013).
The Sandberg Member was proposed for marginal transgressive deposits of the Upper Langhian of the Vienna Basin ( et al., 1994). Limestones were deposited in a circalittoral or infralittoral environment, on rises protected from terrigenous input ( et al., 1994). Although terrestrial ecosystems suggest a subtropical climatic zone for these deposits, the marine microfauna indicates a cooling of the environment compared to the rest of the Langhian period ( et al., 2005).
Heterozoan carbonates of the Pleistocene sequences of Castelluccio are largely dominated by bryozoans and coralline algae. The fossil association suggests a cool-temperate climate, probably just slightly colder than the modern Mediterranean's.
Fossil samples have a remarkably similar vegetative anatomy, without significant variation in thallus organization
or the range and average size of vegetative cells (Table 2;
Fig. 2 ![]() ).
).
Reproductive anatomy is also rather uniform among fossil samples (Table 2; Fig. 3 ![]() ). Both the total range and the average (± s.d.) interval overlap consistently, for both conceptacle diameter and conceptacle height (Fig. 5
). Both the total range and the average (± s.d.) interval overlap consistently, for both conceptacle diameter and conceptacle height (Fig. 5 ![]() ). Pleistocene specimens have the largest conceptacles among all the fossils, while those from the Tertiary Piedmont Basin have the smallest (Table 2;
Fig. 5
). Pleistocene specimens have the largest conceptacles among all the fossils, while those from the Tertiary Piedmont Basin have the smallest (Table 2;
Fig. 5 ![]() ). Conceptacle H/D ratio is
very consistent among the samples and most of the average values lie between 0.33 and 0.34,
i.e., conceptacle with diameter three times their height (Table 2). Only the sample from the upper part of the
Lower Langhian of Lopadea Veche (Romania) is characterized by a slightly higher value of H/D ratio, due to its slightly higher than average conceptacle chambers (Table 2). ANOVA testing
of this morphological parameter confirms that there is very little variation among the different samples and that they
probably belong to the same statistical population.
). Conceptacle H/D ratio is
very consistent among the samples and most of the average values lie between 0.33 and 0.34,
i.e., conceptacle with diameter three times their height (Table 2). Only the sample from the upper part of the
Lower Langhian of Lopadea Veche (Romania) is characterized by a slightly higher value of H/D ratio, due to its slightly higher than average conceptacle chambers (Table 2). ANOVA testing
of this morphological parameter confirms that there is very little variation among the different samples and that they
probably belong to the same statistical population.
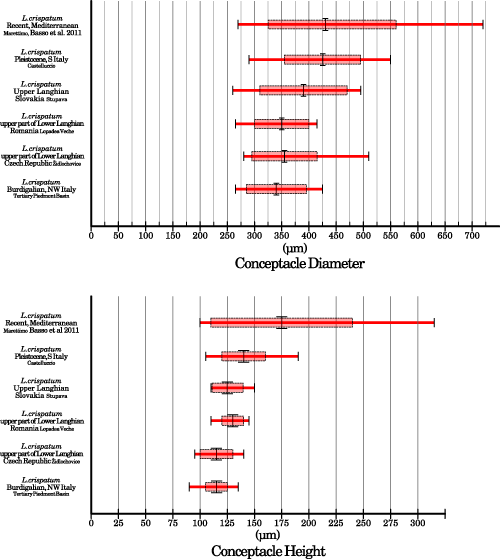
Click on thumbnail to enlarge the image.
Figure 5: Comparison of conceptacle chamber dimensions between fossil and recent samples. The long red bar indicates total range; the pale red rectangle indicates the standard deviation around the average which is represented by the large vertical black bar.
Besides conceptacle size, all the other features of the reproductive anatomy of the fossil samples are remarkably similar. The number of cells that compose the roof and roof thickness are uniform. Large conceptacles, created by the merging of two nearby conceptacles, were observed in all the samples (Table 2).
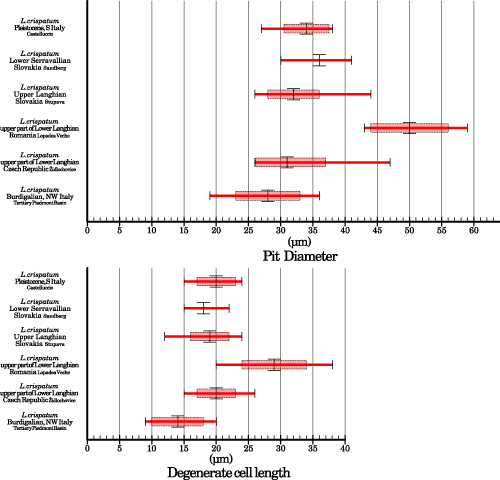
Some differences may be observed in the morphology of roof pits (Fig. 3.G-H ![]() ). While the number of cells composing the rosette around the pore canals in the examined specimens is always 6 to 7, the diameter of the pits and the length of the
degenerate cells are quite variable (Table 2;
Fig. 6
). While the number of cells composing the rosette around the pore canals in the examined specimens is always 6 to 7, the diameter of the pits and the length of the
degenerate cells are quite variable (Table 2;
Fig. 6 ![]() ). Most of the samples, especially those of the Tertiary Piedmont Basin, have small and shallow pits (Fig.
3.A, G
). Most of the samples, especially those of the Tertiary Piedmont Basin, have small and shallow pits (Fig.
3.A, G ![]() ), but the one
from the upper part of the Lower Langhian of Romania has larger and deeper pits (Figs.
3.C, H
), but the one
from the upper part of the Lower Langhian of Romania has larger and deeper pits (Figs.
3.C, H ![]() & 6
& 6 ![]() ). These differences cannot be related to differential preservation of the structures. Although mechanical and biological abrasion of coralline surfaces are common, the Romanian sample shows preserved walls between the cells forming the rosettes (Fig.
3.H
). These differences cannot be related to differential preservation of the structures. Although mechanical and biological abrasion of coralline surfaces are common, the Romanian sample shows preserved walls between the cells forming the rosettes (Fig.
3.H ![]() ),
so that abrasion processes must be ruled out. The internal details of these structures are very well preserved and
it is possible to distinguish between the cavity of the central pore channel and the cavities of the surrounding
degenerate cells (Fig.
3.C
),
so that abrasion processes must be ruled out. The internal details of these structures are very well preserved and
it is possible to distinguish between the cavity of the central pore channel and the cavities of the surrounding
degenerate cells (Fig.
3.C ![]() ). Differential dissolution would have resulted in blurred structures and therefore this process is also unlikely.
). Differential dissolution would have resulted in blurred structures and therefore this process is also unlikely.
Click on thumbnail to enlarge the image.
Figure 6: Comparison of the size of roof pits between fossil samples. The long red bar indicates total range; the pale red rectangle indicates the standard deviation around the average which is represented by the large vertical black bar.
In the dendrogram (Fig. 4 ![]() ) the Romanian sample is separated from all other fossils indicating that, even setting aside the roof pits, this sample is
somewhat different from the others. However, taking in to account the other morphological variables, there is still an almost complete overlap between the ranges and the averages of this sample and those of the remaining fossils (Table 1). Since the differences are largely overwhelmed by the similarities and no
comprehensive information exists on roof-pit morphological variability in modern
L. crispatum, the Romanian specimens are considered conspecific with the other
fossils examined.
) the Romanian sample is separated from all other fossils indicating that, even setting aside the roof pits, this sample is
somewhat different from the others. However, taking in to account the other morphological variables, there is still an almost complete overlap between the ranges and the averages of this sample and those of the remaining fossils (Table 1). Since the differences are largely overwhelmed by the similarities and no
comprehensive information exists on roof-pit morphological variability in modern
L. crispatum, the Romanian specimens are considered conspecific with the other
fossils examined.
In addition to the fossils discussed above, another specimen studied by Harlan (1962, 1964) deserves attention. The specimen is Eocene in age and was collected in the island of Ishigaki (Ryukyu, southern Japan). It was initially misidentified as Lithothamnion vaughanii , 1919 (, 1962), and later as Mesophyllum vaughanii. The published picture shows two conceptacles with clearly pitted roof (, 1962, Pl. 13, fig. 1; , 1964, Pl. 6, fig. 6). According to 's description, the specimen has perithallial cells 10 to 19 μm in length and 8 to 11 μm in diameter, and conceptacles are 191 to 330 μm in diameter and 102 to 132 μm in height (, 1964). The vegetative anatomy is similar to that of the other fossils considered here, but its conceptacles have a significantly smaller diameter. Since a revision of 's material is beyond the scope of this work it is impossible to assess with confidence its conspecificity with L. crispatum as circumscribed in this paper. However, we suggest that multiporate conceptacles with pitted roofs could have appeared as early as the Eocene.
According to and
(1995), et al. (2000), et al.
(2010) and et al.
(2011), the presence of pore canals bordered by a rosette of
degenerate cells is a diagnostic feature that is unique to the genus Lithothamnion.
It allows the identification of Lithothamnion crispatum, so that the fossil specimens examined must be included in this species. Setting aside this key feature, an accurate and direct comparison with the Mediterranean specimens (Marettimo, Egadi Islands, southern Tyrrhenian Sea) reveals that the
fossil and modern groups are morphologically extremely similar (Figs. 7 ![]() - 8
- 8 ![]() ). The vegetative anatomy is almost
identical in both ranges and average values (Table 2;
Fig. 7
). The vegetative anatomy is almost
identical in both ranges and average values (Table 2;
Fig. 7 ![]() ). Reproductive anatomy is also similar: the conceptacles of
modern L. crispatum are similar in size to the fossil ones, and so are the H/D ratio and the roof characteristics (Table 2;
Fig. 8
). Reproductive anatomy is also similar: the conceptacles of
modern L. crispatum are similar in size to the fossil ones, and so are the H/D ratio and the roof characteristics (Table 2;
Fig. 8 ![]() ). The multivariate statistical analysis further
emphasizes the similarity between Recent and fossil L. crispatum, since the two groups are clustered at more than 90% of B-C similarity. On the other hand,
it must be recognized that the multivariate analysis separates the sample from Marettimo from its fossil counterparts (Fig. 4
). The multivariate statistical analysis further
emphasizes the similarity between Recent and fossil L. crispatum, since the two groups are clustered at more than 90% of B-C similarity. On the other hand,
it must be recognized that the multivariate analysis separates the sample from Marettimo from its fossil counterparts (Fig. 4 ![]() ). This separation is caused by the larger average size of the conceptacles of the
Recent specimens. The largest difference occurs between the lower Burdigalian samples of the Tertiary Piedmont Basin and the Marettimo sample, while the Pleistocene sample from Castelluccio is the closest to the modern one (Table 2;
Fig. 5
). This separation is caused by the larger average size of the conceptacles of the
Recent specimens. The largest difference occurs between the lower Burdigalian samples of the Tertiary Piedmont Basin and the Marettimo sample, while the Pleistocene sample from Castelluccio is the closest to the modern one (Table 2;
Fig. 5 ![]() ). However, since the total ranges of the two group overlaps completely, these differences should be considered within the natural variability of the species (Fig. 5
). However, since the total ranges of the two group overlaps completely, these differences should be considered within the natural variability of the species (Fig. 5 ![]() ). The outcome of the analysis of variance performed on the H/D ratio also strengthen this hypothesis. The Marettimo specimens and the fossils also share
similar morphology and a common diameter of the roof pits, length of the
degenerate cells, and number of cells in the rosette (Fig.
8.C-D
). The outcome of the analysis of variance performed on the H/D ratio also strengthen this hypothesis. The Marettimo specimens and the fossils also share
similar morphology and a common diameter of the roof pits, length of the
degenerate cells, and number of cells in the rosette (Fig.
8.C-D ![]() ). Large roof pits, as large as those of the sample from Romania, were observed in the lectotype from the Adriatic Sea, Mediterranean ( et al.,
2011,
Figs. 13-14), suggesting that the size of the roof pits is a quite variable feature.
). Large roof pits, as large as those of the sample from Romania, were observed in the lectotype from the Adriatic Sea, Mediterranean ( et al.,
2011,
Figs. 13-14), suggesting that the size of the roof pits is a quite variable feature.
Click on thumbnail to enlarge the image.
Figure 7: Comparison of the vegetative anatomy of fossil and Recent L. crispatum. (A) Zoned branch, Recent, Mediterranean, Egadi Islands, Marettimo. (B) Zoned branch, Upper Langhian, Slovakia, Stupava-Vrchná Hora. (C) Plumose ventral-core, L. crispatum lectotype, Recent, Rovigno, Mediterranean, Northern Adriatic, modified after et al. (2011). (D) Plumose ventral-core, Upper Langhian, Slovakia, Stupava-Vrchná Hora.
Click on thumbnail to enlarge the image.
Figure 8: Comparison of the reproductive anatomy of fossil and recent L. crispatum. (A) Conceptacle chamber, Recent, Mediterranean, Egadi Islands, Marettimo. (B) Conceptacle chamber, Burdigalian, NW Italy, Tertiary Piedmont Basin, Villa San Bartolomeo. (C) Rosette of degenerate cells around pore canals, Burdigalian, NW Italy, Tertiary Piedmont Basin, Torre Veglio. (D) Rosette of degenerate cells around pore canals, Recent, Mediterranean, Egadi Islands, Marettimo.
A general comparison with data from other papers on modern L. crispatum is more difficult since biometric data are usually provided just as total range, and not all the variables are measured every time. However, it should be pointed out that although all the modern specimens are grouped together by the presence of roof pits, minor morphological differences seem to exist amongst them ( et al., 2011, Table 1). Therefore, the variability displayed by the different populations of L. crispatum in the world's oceans is comparable to, if not greater than, the variability observed amongst the various fossil populations.
Since no morphological evidence can be used to separate modern L. crispatum from the fossil corallines analyzed in this work, they must be considered conspecific, and consequently the stratigraphic range of L. crispatum is extended at least to the Burdigalian.
Fossil and modern specimens share the same vegetative and reproductive anatomy and are characterized by the same unique diagnostic feature: the presence of a rosette of degenerate cells (a pit in axial sections) around the multiporate conceptacle pore canals. Both qualitative comparison and statistical analyses show that their morphology is remarkably similar. The fossil specimens analyzed here are thus conspecific with the modern Lithothamnion crispatum, whose stratigraphic range is extended back at least to the Burdigalian. The species has been found associated with markedly different skeletal assemblages and in very varied climatic conditions throughout the Cenozoic. The fossil record, consistent with its modern cosmopolitan distribution, suggests a remarkable adaptability. Although the morphofunctional significance of L. crispatum roof pits remains unknown, they seem to be a successful and persistent feature.
We are indebted to two anonymous referees who helped us to improve the quality of the manuscript through their detailed comments. We also would like to thank all the participants of the IFAA symposium in Japan for advice and fruitful discussions.
J., M.C. & T.W. (1984).- Estudios en las algas Corallinaceae (Rhodophyta) de las Islas Canarias. Aspectos metodologicos.- Vieraea, Santa Cruz de Tenerife, vol. 13, p. 113-125.
J., J.C., B. & W.J. (2012).- Reassessment of 's newly discovered types of fossil corallines (Corallinales, Rhodophyta) preserved at the Muséum national d'Histoire naturelle, Paris.- Cryptogamie Algologie, Paris, vol. 33, nº 3, p. 289-326.
J., R. & J.C. (2000).- Late Cretaceous incident light reduction: evidence from benthic algae.- Lethaia, Oslo, vol. 33, p. 205-213.
I., A. & M. (1994).- Sandberské vrstvy – vrchnobádenské marginálne sedimenty východného okraja Viedenskej panvy.- Geologické práce, Správy, Bratislava, vol. 99, p. 59-66.
D., J.C. & Y. (2009).- Palaebiogeographic pattern of a persistent monophyletic lineage: Lithophyllum pustulatum species group (Corallinaceae, Corallinales, Rhodophyta).- Palæogeography, Palæoclimatology, Palæoecology, vol. 284, p. 237-245.
D., P. & G. (1996).- Fossil and living corallinaceans related to the Mediterranean endemic species Lithophyllum racemus () .- Facies, Erlangen, vol. 35, p. 275-292.
D., P. & G. (1997).- The taxonomy of Lithothamnion ramosissimum ( non ) and Lithothamnion operculatum (Rhodophyta, Corallinaceae).- Facies, Erlangen, vol. 37, p. 167-182.
D., G. & G. (2011).- A re-description of Lithothamnion crispatum and the status of Lithothamnion superpositum (Rhodophyta, Corallinales).- Phycologia, Lawrence, vol. 5, nº 2, p. 144-155.
G. (1885).- Flora veneta crittogamica; Parte II.- Padova, 255 p.
J.C., D.W.J. & R.S. (1993).- New anatomical characteristics in fossil coralline algae and their taxonomic implications.- Paleontology, London, vol. 36, p. 535-547.
G. & L. (2003).- Corallinales del Mar Mediterraneo: Guida alla determinazione.- Biologia Marina Mediterranea, Genova, vol. 10, 237 Pp.
K.R. & R.N (2006).- PRIMER v6: User Manual/Tutorial.- PRIMER-E, Plymouth, 192 p.
G., D., A. & C. (2015).- Transported rhodoliths witness the lost carbonate factory: a case history from the Miocene Pietra da Cantoni limestone (NW Italy).- Rivista Italiana di Paleontologia e Stratigrafia, Milano, vol. 121, p. 345-368.
A. (1960).- The divisions and classes of plants.- The Botanical Review, New York, vol. 26, p. 425-482.
J., M., B., E., B., M.F., J.P., S. & M. (2000).- Atlas peri-Tethys, palaeogeographical maps.- CCGM-CGMW, Paris, 269 p.
N., R., Š. & S. (2008).- The red–algal facies of the Lower Badenian limestones of the Carpathian Foredeep in Moravia (Czech Republic).- Geologica Carpathica, Bratislava, vol. 59, nº 2, p. 133–146.
N., K., S., Š., R., K., J., M. & T. (2014).- The Badenian parastratotype at Židlochovice from the perspective of the multiproxy study.- Neues Jahrbuch für Geologie und Paläontologie, Stuttgart, vol. 271, nº 2, p. 169-201.
J.G., K.R. & R.M. (1982).- A practical strategy for analyzing multispecies distribution patterns.- Marine Ecology Progress Series, Hamburg, vol. 8, p. 37-52.
S. & R. (1997).- Lower Badenian Sea-Level Drop on the Western Border of the Transylvanian Basin: Foraminiferal Paleobathymetry and Stratigraphy.- Geologica Carpathica, Bratislava, vol. 48, nº 5, p. 325-334.
J.E. (1864).- Handbook of British water-weeds or algae.- R. Hardwicke, London, 123 p.
A.S., S.T., W.J. & P.J. (2003).- Choreonema (Corallinales, Rhodophyta): 18S rDNA phylogeny and resurrection of the Hapalidiaceae for the subfamilies Choreonematoideae, Austrolithoideae, and Melobesioideae.- Journal of Phycology, Moss Landing, vol. 39, p. 988-998.
F. (1878).- Beirträge zur Kenntniss der Adriatischen Algen X.- Österreichische Botanische Zeitschrift, Wien, vol. 28, p. 288-295.
T.G., C. & W.E. (1997).- Benthic foraminiferal associations in the Northern Bay of Safaga, Red Sea, Egypt.- Marine Micropaleontology, vol. 29, p. 185-210.
F. (1897).- Melobesiae.- Berichte der deutsche botanischen Gesellschaft, Berlin, vol. 15, p. 403-420.
M.A. (1919).- On some fossil and recent Lithothamnieae of the Panama Canal zone.- United States National Museum Bulletin, Washington, D.C., vol. 103, p. 1-13.
J., D. & N. (2015).- Diagnostic characters in fossil coralline algae (Corallinophycidae: Rhodophyta) from the Miocene of southern Moravia (Carpathian Foredeep, Czech Republic).- Journal of Systematic Paleontology, London, DOI: 10.1080/14772019.2015.1071501.
J. & K. (2013).- Paleoecological evaluation of the algal limestones in Stupava-Vrchná Hora locality (Vienna Basin, Slovakia).- Mineralia Slovaca, Košice, vol. 45, p. 11-22.
Y., D. & W.J. (2009).- Re-Assessment of the type collections of fourteen corallinalean species (Coralline, Rhodophyta) described by W. (1942-1960).- Paleontology, London, vol. 52, nº 2, p. 401-427.
J.H. (1962).- The algal genus Lithothamnion and its fossil representatives.- Colorado School of Mines Quaterly, Golden, vol. 57, 111 p.
J.H. (1964).- Eocene Algae from Ishigaki-shima, Ryūkyū-rettō.- Geological Survey Professional Paper, Washington, D.C., vol. 399-C, p. 1-13.
D.W., G. & Y.M. (2000).- Lithothamnion superpositum : a common crustose red alga (Corallinaceae) in South Africa.- Cryptogamie Algologie, Paris, vol. 21, nº 4, p. 381-400.
M., K., A.S., E., N., P., R., M., M. & Ľ. (2005).- Západokarpatské fosílne ekosystémy a ich vzťah k paleoprostrediu v kontexte neogénneho vývoja eurázijského kontinentu.- Geologické práce, Správy, Bratislava, vol. 111, p. 61-121.
J.B. (1977).- Multidimensional scaling and other methods for discovering structure. In: A. & H.S. (eds.), Statistical methods for digital computers.- Wiley, New York, p. 296-339.
L. & G.W. (2007).- A nuclear phylogeny of the Florideophyceae (Rhodophyta) inferred from combined EF2, small subunit and large subunit ribosomal DNA: establishing the new red algal subclass Corallinophycidae.- Molecular Phylogenetics and Evolution, vol. 43, p. 1118-1130.
M., T. & D.L. (2005).- Cenozoic continental climatic evolution of Central Europe.- Proceedings of the National Academy of Science of the United States of America, Washington, D.C., vol. 102, nº 42, p. 14964-14969.
K.F., W.A., R., D. & T.J. (2015).- Northern New Zealand rhodoliths: assessing faunal and floral diversity in physically contrasting beds.- Marine Biodiversity, vol. 45, p. 63-75.
W.A., J.E., T.J., D.R., K.F., H.J. & H.S. (2015).- Multi-gene phylogenetic analyses of New Zealand coralline algae Corallinapetra novaezelandiae gen. et sp. nov. and recognition of the Hapalidiales ord. nov.- Journal of Phycology, Moss Landing, vol. 51, p. 454-468.
J., R., Z., E.C. & P.A. (2010).- Lithothamnion superpositum (Corallinales; Rhodophyta): First description for the Western Atlantic or rediscovery of a species?.- Phycological Research, vol. 58, p. 210-216.
C., P., R., F., M., J.M., E.C. & P. (2013).- Seasonal and depth-driven changes in rhodolith bed structure and associated macroalgae off Arvoredo Island (Southeastern Brazil).- Aquatic Botany, vol. 111, p. 62-65.
F., G. & D. (2007).- Neogoniolithon contii comb nov based on the taxonomic re-assessment of 's original material from the Oligocene of NW Italy (TPB).- Rivista Italiana di Paleontologia e Stratigrafia, Milano, vol. 113, nº 1, p. 43-55.
M.W. & W.E. (1999).- Application of neontological taxonomic concepts to Late Eocene coralline algae (Rhodophyta) of the Austrian Molasse Zone.- Journal of Micropaleontology, London, vol. 18, nº 1, p. 67-80.
M.W. & W.E. (2000).- Designation of Phymatolithon (Corallinaceae, Rhodophyta) in fossil material and its paleoclimatological indications.- Micropaleontology, New York, vol. 46, nº 1, p. 89-95.
J.-Y. & N.P. (2012).- The Miocene Sommičres basin, SE France: Bioclastic carbonates in a tide-dominated depositional system.- Sedimentary Geology, vol. 282, p. 360-373.
J.P., D., J.J., S., S. & I.I. (2007).- Les constructions coralliennes du Badénien (Miocčne moyen) de la bordure occidentale de la depression Transylvanie (Roumanie).- Comptes Rendus Palevol, Paris, vol. 6, p. 37-46.
P.C., P.W. & R.L. (1996).- Catalogue of the benthic marine algae of the Indian Ocean.- University of California Press, Berkeley, 1129 p.
G., M., C. & C. (1996).- Litostratigrafia e paleoecologia di successioni a rodoliti della Pietra da Cantoni (Monferrato Orientale, Italia Nord-Occidentale).- Atti Societŕ Toscana di Scienze Naturali, Memorie, Pisa, (Seria A), vol. 103, p. 69-86.
G., F. & D. (2008).- Revision and redocumentation of M. species of Lithophyllum from the Tertiary Piedmont Basin.- Rivista Italiana di Paleontologia e Stratigrafia, Milano, vol. 114, nº 3, p. 515-528.
K.M. & W.J. (1995).- An account of Southern Australian species of Lithothamnion (Corallinaceae, Rhodophyta).- Australian Systematic Botany, Clayton South, vol. 8, p. 549-583.
W.J., B. & D. (2014).- Heydrichia (?) poignantii, sp. nov. (Sporolithaceae, Sporolithales, Rhodophyta), a 100 million year old fossil coralline red alga from north-eastern Brazil, and a new Hauterivian record of Sporolithon from Switzerland.- Carnets Geol., Madrid, vol. 14, nº 7, p. 139-158.
W.J., L.M. & A.S. (1993).- Growth-forms in non-geniculate coralline red algae (Corallinales, Rhodophyta).- Australian Systematic Botany, Clayton South, vol. 6, p. 277-293.