◄ Carnets Geol. 16 (10) ►
Contents
[1. Introduction]
[2. Geological setting]
[3. Material and mthods]
[4. Results]
[5. Discussion] and ...
[Bibliographic references]
University of Silesia,
Faculty of Earth Sciences, Będzińska Str. 60, PL-41-200 Sosnowiec (Poland);
University of
Silesia, Faculty of Earth Sciences - Centre for Polar
Studies KNOW (Leading National Research Centre), Będzińska Str. 60,
PL-41-200 Sosnowiec (Poland)
University of Wrocław, Faculty of Earth Sciences and Environmental Management, The Institute of Geological Sciences, plac Maksa Borna 9, PL-50-205 Wrocław (Poland)
University of Silesia,
Faculty of Earth Sciences, Będzińska Str. 60, PL-41-200 Sosnowiec (Poland);
University of
Silesia, Faculty of Earth Sciences - Centre for Polar
Studies KNOW (Leading National Research Centre), Będzińska Str. 60,
PL-41-200 Sosnowiec (Poland)
Dame du Lac 213, 3 rue Henri Barbusse,
F-76300 Sotteville-lès-Rouen (France)
University of Silesia,
Faculty of Earth Sciences, Będzińska Str. 60, PL-41-200 Sosnowiec (Poland);
University of
Silesia, Faculty of Earth Sciences - Centre for Polar
Studies KNOW (Leading National Research Centre), Będzińska Str. 60,
PL-41-200 Sosnowiec (Poland)
Published online in final form (pdf) on May 21, 2016
[Editor: Bruno Granier;
language editor: Donald Owen]
Fossil shells of the marine bivalve Plagiostoma striatum Schlotheim sampled from the Middle Triassic (so-called Muschelkalk) of Poland demonstrate that, under unfavourable environmental conditions, this species commonly occurring in Triassic German basins exhibits a dwarfed shell. As a consequence of a marine regression episode resulting in a significant increase of salinity and a partial emersion of seafloor these bivalves vanished. The next transgressive pulse caused a re-emergence of these bivalves. They were initially characterized by half-size shells than in the population living prior to the regression episode and, subsequently, during progressive transgression, their shells returned to normal size. Coincidence between eustatic curve and changes in bivalve shell size and their disappearance may be attributed also to biotic interactions, such as a biotic collapse in primary bioproductivity or/and a competition for space or any other resources due to shelf habitat loss during regressive periods.
Bivalvia; Plagiostoma striatum; Triassic; Lilliput effect; sea level fluctuations.
Brom K.R., Niedźwiedzki
R., Brachaniec T, Ferré
B. & Salamon M.A. (2016).- Environmental control
on shell size
of Middle Triassic bivalve Plagiostoma.- Carnets Géol., Madrid, vol.
16, nº 10, p. 297-305.
Contrôle environnemental de la taille des coquilles chez Plagiostoma, un bivalve du Trias moyen.- Des coquilles fossiles du bivalve marin Plagiostoma striatum Schlotheim échantillonnées dans le Trias moyen (communément appelé Muschelkalk) de Pologne montrent que, dans des conditions environnementales défavorables, cette espèce courante dans les bassins du Trias germanique présente une taille réduite. Suite à un épisode régressif marin s'exprimant par une augmentation significative de la salinité et une exondation partielle de la partie proximale de la plate-forme et des environnements les moins profonds, ce lamellibranche disparaît. Initialement caractérisées par une taille réduite de moitié par rapport aux populations antérieures à l'épisode régressif, par la suite, leurs coquilles reviennent à une taille normale progressivement au cours de la transgression suivante. La corrélation entre la courbe eustatique et les changements de taille de la coquille de ces bivalves (et leur disparition) peut être également attribuée à des interactions biotiques, telles qu'un effondrement de la productivité biologique primaire et/ou à une compétition pour l'espace trophique ou pour toute autre ressource liée à une perte d'habitat sur la plate-forme lors de périodes régressives.
Bivalves ; Plagiostoma striatum ; Trias ; effet Lilliput ; variations eustatiques.
Adaptations of organisms to permanently changed environmental conditions or environmental stress are one of the key factors of evolution. These features could be accomplished by changing their structure (morphology and/or size) or their life functions (Futuyma, 2008). Currently, there are several "rules" relating to changes in the volume of individual organisms over geologic time because size is the most recognizable attribute in the fossil record and it is commonly used in describing evolutionary trends (Urbanek, 1993; Twitchett, 2006; Harries & Knor, 2009; Wade & Twitchett, 2009; Huang et al., 2010; Borths & Ausich, 2011).
One of them, the Lilliput Effect, also called the "anti-Cope's Rule", refers to decrease in body size of organisms in response to biotic events, during which environmental conditions have significantly deteriorated to generate a mass extinction. Generally three patterns of Lilliput Effect are addressed: 1) extinction of large taxa, 2) within-lineage size decrease, and 3) miniaturization. Some authors suggest that a fourth pattern may also occur - decrease in average body size with post-extinction Lazarus gaps (Harries & Knor, 2009; Huang et al., 2010; Borths & Ausich, 2011). The Lilliput Effect as an adaptation allows organisms to accelerate the onset of sexual maturity and thus to optimize opportunities for earlier reproduction. The affected organisms require fewer calories for growth and function, which is highly advantageous in a less-stable environment with decreased food availability (Borths & Ausich, 2011). The Lilliput Effect could be induced by the following factors: a biotic collapse in primary productivity, physicochemical changes in environmental conditions (e.g., salinity, temperature), a loss of symbionts and sea-level fluctuations (Lockwood, 2005; Twitchett, 2006; Wade & Peterson, 2008; Wade & Twitchett, 2009; Borths & Ausich, 2011). These changes have a direct and/or indirect impact on living organisms, forcing them to adapt to changing environment (Benton, 2009). One of the major reasons affecting body size in marine animals is the onset of anoxic events (OAEs), e.g., Wade and Twitchett (2009); Borths and Ausich (2011); Brom et al. (2015b). Several such events were recorded during the Mesozoic, though none of them were documented in the Middle Triassic (Hallam, 1997).
Borths and Ausich (2011) described decrease in average body size with post-extinction Lazarus gaps. Originally the Lazarus Effect is defined as the temporary disappearance of taxa from the fossil record in any given time interval (Fara, 2001). However, many authors consider the Lazarus Effect as a pattern restricted to mass extinction episodes (Jablonski, 1986; Fara, 2001), although some other authors, when connecting Lazarus Effect phenomenon with episodes of mass extinction, use the term "dead clade walking" (Salamon et al., 2010; Gorzelak et al., 2011). On the other hand, Twitchett (2006) suggested using the term "Refugia taxa" instead of "Lazarus taxa" due to a low probability of settling new habitats or regions by species during biotic crises, in order to avoid them.
The present study aims to introduce an initial investigation on Muschelkalk bivalves Plagiostoma striatum Schlotheim which support the Lilliput Effect, as well as the Lazarus Effect. These phenomena occurred as a side-effect of sea-level changes and are likely an adaptative response to changing environmental conditions.
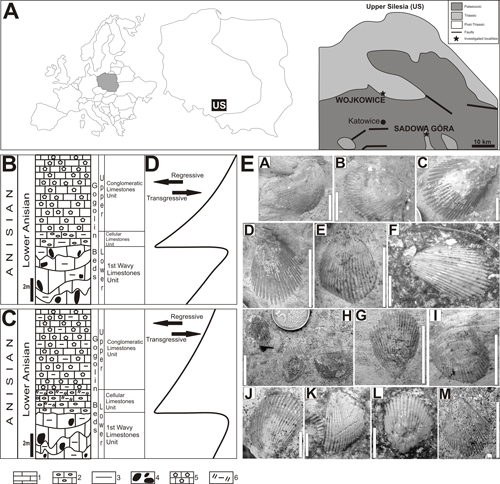
Abandoned exposures of studied Triassic limestones in the Wojkowice and Sadowa Góra quarries are located in Upper Silesia, southern Poland. These quarries are windows on the Middle Triassic deposits of the Silesian-Cracow Monocline. In both quarries, the lowest part of the Lower Muschelkalk is mainly represented by limestones and marly limestones of the so-called Lower and Upper Gogolin Beds. Based on conodont investigation and magnetostratigraphic studies, the Gogolin Beds had been deposited in the latest Olenekian - early Pelsonian (Zawidzka, 1975; Nawrocki & Szulc, 2000). The Gogolin Beds were formed in a carbonate ramp environment, during two marine transgressive phases (Szulc, 1993). The first stage, with a normal marine salinity, was responsible for the formation of the Entolium and Dadocrinus limestones unit and the overlying 1st Wavy Limestones unit (1st Wellenkalk unit). Subsequently, a brief regression episode occurred. As a by-product of the regression, the dolomitic limestones and dolomites of the Cellular Limestones unit (Zellenkalk 2 unit) were formed. Finally, the Conglomeratic Limestones unit was deposited at the beginning of the transgressive phase, which also produced the upper lithostratigraphic units of the Upper Gogolin Beds (Szulc, 1993).
2.1. Sadowa Góra quarry
The
Sadowa Góra quarry is located 2 km north of Jaworzno City, south Upper Silesia
(Fig. 1.A ![]() ). The lithological profile is ca. 30 m high and presents the Lower and
Upper Gogolin Beds. The lower lithological unit of the Sadowa Góra profile is
represented mainly by a yellow- and grey, and light-grey coloured, fine-grained
limestones with Entolium and Dadocrinus. Locally, this limestone contains minor glauconite and
marly interbeds. A typical feature is the occurrence of numerous Entolium
discites (Schlotheim) and Dadocrinus
columnals. The overlying 1st Wavy Limestones unit displays a medium lumpy
structure, grey, pelitic limestone and marly interbeds, and less scarce fauna
than in the underlying limestone (Fig. 1.B
). The lithological profile is ca. 30 m high and presents the Lower and
Upper Gogolin Beds. The lower lithological unit of the Sadowa Góra profile is
represented mainly by a yellow- and grey, and light-grey coloured, fine-grained
limestones with Entolium and Dadocrinus. Locally, this limestone contains minor glauconite and
marly interbeds. A typical feature is the occurrence of numerous Entolium
discites (Schlotheim) and Dadocrinus
columnals. The overlying 1st Wavy Limestones unit displays a medium lumpy
structure, grey, pelitic limestone and marly interbeds, and less scarce fauna
than in the underlying limestone (Fig. 1.B ![]() ). The next overlying one is the Cellular
Limestones unit with dolomitic limestones and marl intercalations. No fossil has been
found within during field investigations. The lower part
of the Upper Gogolin Beds is the Conglomeratic Limestones unit, consisting of
light-grey, pelitic limestone intraclasts (length 0.5 cm to 9 cm) in an
organodetrital matrix. Among the numerous shelly fauna, bivalves, mainly Plagiostoma and Entolium,
rare disarticulated shells of
brachiopod Coenothyris vulgaris Schlotheim,
gastropods, foraminifers and ichnofossils (Palaeophycus)
were identified. Lying on the conglomerate level, the 2nd Wavy Limestones unit
consists of several layers of pelitic limestone distinctly showing a wavy-bedded
texture. The above-lying Marly Limestone unit was formed in a similar way;
however, a wavy-bedded texture does not occur here. The highest horizon of the
Gogolin Beds in the Sadowa Góra quarry is the 3rd Wavy Limestones unit that
underwent dolomitization processes and is a part of the so-called ore-bearing
dolomites (Bojkowski, 1955). Both fossils and
sedimentary structures
were faded away by secondary metasomatic processes. The fine-grained, yellow
and grey dolomite can reach 4 m of thickness, intercalated between clay and
dolomitized marlstone interbeds.
). The next overlying one is the Cellular
Limestones unit with dolomitic limestones and marl intercalations. No fossil has been
found within during field investigations. The lower part
of the Upper Gogolin Beds is the Conglomeratic Limestones unit, consisting of
light-grey, pelitic limestone intraclasts (length 0.5 cm to 9 cm) in an
organodetrital matrix. Among the numerous shelly fauna, bivalves, mainly Plagiostoma and Entolium,
rare disarticulated shells of
brachiopod Coenothyris vulgaris Schlotheim,
gastropods, foraminifers and ichnofossils (Palaeophycus)
were identified. Lying on the conglomerate level, the 2nd Wavy Limestones unit
consists of several layers of pelitic limestone distinctly showing a wavy-bedded
texture. The above-lying Marly Limestone unit was formed in a similar way;
however, a wavy-bedded texture does not occur here. The highest horizon of the
Gogolin Beds in the Sadowa Góra quarry is the 3rd Wavy Limestones unit that
underwent dolomitization processes and is a part of the so-called ore-bearing
dolomites (Bojkowski, 1955). Both fossils and
sedimentary structures
were faded away by secondary metasomatic processes. The fine-grained, yellow
and grey dolomite can reach 4 m of thickness, intercalated between clay and
dolomitized marlstone interbeds.
2.2. Wojkowice quarry
The
Wojkowice quarry (Fig. 1.A ![]() )
displays Rhaetian (Röt) formations and a nearly
complete record of the Gogolin Beds. Thickness of these latter is ca. 30 m. The
lower part of the Lower Gogolin Beds is represented by organodetrital
limestones, consisting of a large number of Entolium
discites bivalves and skeletal elements of Dadocrinus
crinoids (Limestones with Entolium and
Dadocrinus unit), as well as
undetermined gastropods and vertebrate remains (e.g., fish scales of Gyrolepis
and shark teeth of Acrodus). Plagiostoma bivalves are very rare. In this unit, Salamon et
al. (2012a) evidenced the occurrence of numerous bromalites. The organodetrital
and crinoidal limestones building the Limestones with Entolium and Dadocrinus
unit in Upper Silesia are interpreted as proximal tempestites (Hagdorn &
Szulc, 2007). Above, occurs a complex of lumpy wavy-bedded limestones
with marl intercalations (1st Wavy Limestones unit) (Fig.
1.C
)
displays Rhaetian (Röt) formations and a nearly
complete record of the Gogolin Beds. Thickness of these latter is ca. 30 m. The
lower part of the Lower Gogolin Beds is represented by organodetrital
limestones, consisting of a large number of Entolium
discites bivalves and skeletal elements of Dadocrinus
crinoids (Limestones with Entolium and
Dadocrinus unit), as well as
undetermined gastropods and vertebrate remains (e.g., fish scales of Gyrolepis
and shark teeth of Acrodus). Plagiostoma bivalves are very rare. In this unit, Salamon et
al. (2012a) evidenced the occurrence of numerous bromalites. The organodetrital
and crinoidal limestones building the Limestones with Entolium and Dadocrinus
unit in Upper Silesia are interpreted as proximal tempestites (Hagdorn &
Szulc, 2007). Above, occurs a complex of lumpy wavy-bedded limestones
with marl intercalations (1st Wavy Limestones unit) (Fig.
1.C ![]() ). In the uppermost
part, the Cellular Limestones unit is visible. In Wojkowice, this unit is
developed as yellow limestones with dolomite and marly dolomite layers. No
bivalve evidence has been found there. The upper part of this unit displays two
concentration beds of ophiuroids (Salamon et al.,
2012b; see also Zatoń
et al., 2008). The thickness of the overlying conglomerate limestones reaches
10 m. This unit displays micrite and organodetrital limestones with intraclasts
of pelitic limestone (length 0.5 cm to 15 cm). Numerous holocrinids and
encrinids, gastropods, vertebrate remains (Salamon et al.,
2012a) and rare
disarticulated brachiopod shells of Coenothyris
vulgaris and Punctospirella fragilis Schlotheim,
echinoid spines, foraminifers, ostracods and ichnofossils (invertebrate
burrows Palaeophycus, Planolites,
Trypanites) were found therein. The highest units in the profile are three
complexes of wavy-bedded and marly limestones (2nd Wavy Limestones unit, Marly
Limestones unit and 3rd Wavy Limestones unit).
). In the uppermost
part, the Cellular Limestones unit is visible. In Wojkowice, this unit is
developed as yellow limestones with dolomite and marly dolomite layers. No
bivalve evidence has been found there. The upper part of this unit displays two
concentration beds of ophiuroids (Salamon et al.,
2012b; see also Zatoń
et al., 2008). The thickness of the overlying conglomerate limestones reaches
10 m. This unit displays micrite and organodetrital limestones with intraclasts
of pelitic limestone (length 0.5 cm to 15 cm). Numerous holocrinids and
encrinids, gastropods, vertebrate remains (Salamon et al.,
2012a) and rare
disarticulated brachiopod shells of Coenothyris
vulgaris and Punctospirella fragilis Schlotheim,
echinoid spines, foraminifers, ostracods and ichnofossils (invertebrate
burrows Palaeophycus, Planolites,
Trypanites) were found therein. The highest units in the profile are three
complexes of wavy-bedded and marly limestones (2nd Wavy Limestones unit, Marly
Limestones unit and 3rd Wavy Limestones unit).
Click on thumbnail to enlarge the image.
Figure 1: A. General maps of Europe, Poland, and Upper Silesia (southern Poland) showing location of investigated sections (map of Upper Silesia from Senkowiczowa, 1973, and Salamon et al. 2012b). B. Simplified lithostratigraphic section of the Sadowa Góra quarry (simplified after Bojkowski, 1955). C. Simplified lithostratigraphic section of the Wojkowice quarry (simplified after Hagdorn & Głuchowski, 1993). 1-limestones, 2-cellular limestones, 3-marls, 4-lumpy structures, 5-conglomeratic limestones, 6-marly dolomites. D. Eustatic curve (simplified after Szulc, 1993, Hagdorn & Głuchowski, 1993, and Szulc, 2000). E. Plagiostoma striatum shells sampled from the Sadowa Góra (A-F) and Wojkowice quarries (H-M). A, B, F, J-L - 1st Wavy Limestones Unit; D, E, H-I - Cellular Limestones Unit; C, M - Conglomeratic Limestones Unit. Scale bar equals 10 mm.
In the Gogolin Beds of Upper Silesia only 4 species of the family Limidae Rafinesque occurred. Two of them are ranging in the entire profile of the Gogolin Beds: Plagiostoma striatum Schlotheim and P. lineatum Schlotheim, whereas Pseudolimea acutecostata Assmann and Lima beyrichi Eck only occur in the Upper Gogolin Beds (Assmann, 1937; Niedźwiedzki, 2002).
For
analytical purposes of shell size changes along the stratigraphic section, we
only considered specimens of P. striatum that were collected from
the most fossiliferous layer of each unit, since two species of Lima
were not found and P. lineatum shells
were found less abundant than those of P.
striatum. Additionally, the number of P.
lineatum specimens was not sufficient to draw statistically reliable
conclusions on the change of trends in shell size along the profile. In both
examined quarries, P. striatum ranges through
the entire lithological profile, except in the Cellular Limestones unit. However,
in the Limestones with Entolium and Dadocrinus
unit, they are rare and many are poorly preserved. They are also very rare in the
2nd Wavy Limestones unit. In the 3rd Wavy Limestones unit, we also found only
few specimens of P. striatum, although
it may result from the poor exposure of this lithostratigraphic unit.
Collection of specimens of P. striatum
(large enough to justify a statistically reliable analysis) was obtained in both
quarries only from the 1st Wavy Limestones unit and the Conglomerate Limestones
unit (Fig. 1 ![]() ). Only well-preserved, undeformed, and sufficiently complete P. striatum shells were used for analysis to perform biometrical
measurements (length and height). All sampled shells were disarticulated. Both
valves (left and right ones) of Plagiostoma
specimens are equal in size and shape (our observations, see also description of
Limidae in Cox et al., 1969),
and both were included in the analysis, in the following proportions, left
valves - from 40% to 54% and right valves - from 46% to 60% from different
levels and different localities (total number of left and right valves: 257
specimens).
). Only well-preserved, undeformed, and sufficiently complete P. striatum shells were used for analysis to perform biometrical
measurements (length and height). All sampled shells were disarticulated. Both
valves (left and right ones) of Plagiostoma
specimens are equal in size and shape (our observations, see also description of
Limidae in Cox et al., 1969),
and both were included in the analysis, in the following proportions, left
valves - from 40% to 54% and right valves - from 46% to 60% from different
levels and different localities (total number of left and right valves: 257
specimens).
The fossils were brought to the Laboratory of the Department of Paleontology and Stratigraphy at the University of Silesia, Katowice (Poland). After gentle washing and additional preparation when necessary, they were measured and photographed. In order to ensure statistically significant differences of height and length between bivalves from the 1st Wavy Limestones unit and the Conglomeratic Limestones unit, we adopted the Mann-Whitney U test with Bonferroni correction, using the PAST 3.0 software (PAleontological STatistics, ver. 3.0, Hammer et al., 2001).
In the 1st Wavy Limestones unit of the Wojkowice quarry, 41 specimens with dimensions ranging from 2.9 to 5.1 cm in height (median: 3.8 cm) and from 2 to 4.6 cm in length (median: 2.9 cm), were collected. In the Sadowa Góra quarry, 37 specimens with dimensions ranging from 2.6 to 5.1 cm in height (median: 3.3 cm) and from 1.8 to 4.3 cm in length (median: 2.6 cm) (height and length measurement of bivalve shell according to Fig. 10 in McRoberts, 2011) were sampled. The Cellular Limestones unit in both quarries contained no bivalves. They were found in the lower part of the Conglomeratic Limestones unit. The Wojkowice quarry provided 43 specimens whose dimensions range from 0.8 cm to 3.8 cm in height (median: 1.9 cm) and from 0.4 cm to 3.1 cm in length (median: 1.2 cm). Similarly, in the Sadowa Góra quarry, we collected 55 bivalves of dimensions ranging from 1.0 cm to 3.1 cm in height (median: 1.8 cm) and from 0.6 cm to 2.3 cm in length (median: 1.2 cm). In the middle and upper parts of the Conglomerate Limestones unit of the Wojkowice quarry, 33 bivalves of dimensions ranging from 2.0 cm to 5.0 cm in height (median: 3.8 cm) and from 1.9 cm to 4.6 cm in length (median: 3.0 cm) were found. Similarly, in the Sadowa Góra quarry, 48 bivalves of dimensions spanning from 2.2 cm to 5.6 cm in height (median: 3.75 cm) and from 1.6 cm to 5.1 cm in length (median: 2.85 cm) were collected (Table 1).
Data analysis (Table 1) shows that P. striatum found in the same lithostratigraphic unit from both examined quarries do not differ significantly from each other in terms of valve size (the sole exception being the height of shells from the 1st Wavy Limestones units, but the median and range sizes are comparable, see Table 2). In both quarries, similar variability in size of P. striatum bivalves along the stratigraphic profile could be observed. Median, minimum and maximum size values (length and height) of P. striatum in the 1st Wavy Limestones unit are similar to those of P. striatum obtained from the middle and upper parts of the Conglomeratic Limestones unit. Scarce undamaged and undeformed shells of these bivalve species from the Limestones with Entolium and Dadocrinus unit and from the 2nd Wavy Limestones unit, and 3rd Wavy Limestones unit, are of similar size. However, P. striatum from the lower part of the Conglomeratic Limestones unit is characterized by valves, about half-sized, compared to specimens collected from other units or horizons. Size of shells in two Conglomeratic units in a given quarry is also different though their size range and median size are comparable.
Table 1: Shell size measurements of bivalve Plagiostoma striatum.
| Sadowa Góra quarry | ||||||
| Height [cm] | Length [cm] | |||||
| Min | Med | Max | Min | Med | Max | |
| 1st Wavy Limestones unit | 2.6 | 3.3 | 5.1 | 1.8 | 2.6 | 4.3 |
| Conglomeratic Limestone unit - lower part | 1.0 | 1.8 | 3.1 | 0.6 | 1.2 | 2.3 |
| Conglomeratic Limestone unit - middle to upper part | 2.2 | 3.75 | 5.6 | 1.6 | 2.85 | 5.1 |
| Wojkowice quarry | ||||||
| Height [cm] | Length [cm] | |||||
| Min | Med | Max | Min | Med | Max | |
| 1st Wavy Limestones unit | 2.9 | 3.8 | 5.1 | 2.0 | 2.9 | 4.6 |
| Conglomeratic Limestone unit - lower part | 0.8 | 1.9 | 3.8 | 0.4 | 1.2 | 3.1 |
| Conglomeratic Limestone unit - middle to upper part | 2.0 | 3.8 | 5.0 | 1.9 | 3.0 | 4.6 |
Table 2: Results of the Mann-Whitney U test with Bonferroni correction.
|
Mann-Whitney U test results for height of P. striatum shells |
||||||
|
|
1st Wavy Limestones unit |
Conglomeratic |
Conglomeratic |
1st Wavy Limestones unit |
Conglomeratic |
Conglomeratic |
|
1st Wavy Limestones unit |
- |
6.703 E-14 |
0.421 |
0.231 |
1.487 E-11 |
1.845 |
|
Conglomeratic L. unit - lower part |
|
- |
2.898 E-16 |
1.624 E-15 |
1.386 |
3.203 E-12 |
|
Conglomeratic L. unit - middle to upper part |
|
|
- |
0.714 |
1.193 E-13 |
1.483 |
|
1st Wavy Limestones unit |
|
|
|
- |
3.408 E-13 |
1.801 |
|
Conglomeratic L. unit - lower part |
|
|
|
|
- |
2.435 E-10 |
|
Conglomeratic L. unit - middle to upper part |
|
|
|
|
|
- |
|
Mann-Whitney U test results for length of P. striatum shells |
||||||
|
|
1st Wavy Limestones unit |
Conglomeratic |
Conglomeratic |
1st Wavy Limestones unit |
Conglomeratic |
Conglomeratic |
|
1st Wavy Limestones unit |
- |
3.255 E-14 |
0.800 |
0.684 |
1.085 E-11 |
1.654 |
|
Conglomeratic L. unit - lower part |
|
- |
4.579 E-16 |
1.385 E-15 |
1.238 |
4.14 E-14 |
|
Conglomeratic L. unit - middle to upper part |
|
|
- |
1.514 |
4.203 E-13 |
0.962 |
|
1st Wavy Limestones unit |
|
|
|
- |
3.535 E-13 |
1.629 |
|
Conglomeratic L. unit - lower part |
|
|
|
|
- |
8.457 E-11 |
|
Conglomeratic L. unit - middle to upper part |
|
|
|
|
|
- |
In both investigated quarries, in the lower part of the Conglomeratic Limestones unit, a P. striatum assemblage consisting of significantly smaller shells than in the upper part of that lithostratigraphic unit and also in other units of the Gogolin Beds occur. Small-sized shells in sampled assemblages could originate from various reasons: selection of material during post-mortem transport, juvenile character of the population or unfavourable ecological conditions leading to dwarfism (e.g., Wade & Twitchett, 2009; Borths & Ausich, 2011). Presence of numerous intraclasts, relatively large amount of biodetritus, disarticulation of brachiopod (Articulata) shells, all evidence a high-energy depositional environment. Despite that, the size of both intraclasts and bioclasts in the lower part of the Conglomeratic Limestones unit show a high degree of variability within the same layer, thus supporting a poor sorting of the material at hand. Furthermore, this latter feature is also supported by the equal number of left and right valves of P. striatum. Moreover, in both quarries, located at a considerable distance from each other (26 km), small P. striatum shells of similar size were found in the same lithostratigraphic horizons. It seems unlikely that the two areas on the carbonate ramp showed the similar transport conditions, favouring the selection of the same shell-size class.
In both P. striatum assemblages (from the lower part of the Conglomeratic Limestones unit in Sadowa Góra and Wojkowice quarries), shells are relatively thick (up to 1.8 mm, a feature hard to find out in other levels) and, on some specimens, several growth rings are visible. Similar number of growth rings can be observed on the larger P. striatum shells from other lithostratigraphic units. This does support that every examined population at hand is not juvenile. Therefore, most likely, the cardinal reason of small-sized shell occurrence in the lower part of the Conglomeratic Limestones unit is unfavourable ecological conditions during the sedimentation of this unit. This led to dwarfing of the population. Examined assemblages of P. striatum bivalves in both quarries are situated directly on the Cellular Limestones unit. The Cellular Limestones unit is associated with short-term, but rapid and drastic changes in sedimentation (Szulc, 2000; Salamon et al., 2012b). Sedimentological, petrographic and geochemical analyses of these sediments in the Upper Silesia, including the Wojkowice quarry (namely the occurrence of standard microfacies types SMF 23 - non-laminated homogeneous micrite and microsparite without fossils - and SMF 25 - laminated evaporite-carbonate mudstone, cellular structures, post-gypsum pseudomorphs, palisadic calcite layer, rauhwacke and dolocrete layers, as well as isotopic data of δ13C, δ18O, a high level of boron concentration (Szulc, 2000; Salamon et al., 2012b), and very scanty fossils (Assmann, 1937; Salamon et al., 2012b; this study) suggested that the Cellular Limestones unit sediments had been formed during a regression episode, even leading to a partial emersion of seafloor (sabkha environment) and increasing salinity to a hypersalinity level (Szulc, 2000; Kowal-Linka, 2010; Salamon et al., 2012b). Later on, in the Conglomeratic Limestones unit, a glut of stenohaline fauna (large number of Dadocrinus and Holocrinus crinoid ossicles, echinoid spines, brachiopods Coenothyris vulgaris and Punctospirella fragilis) supports a return to normal seawater salinity. It should be emphasized that stenohaline fauna only occurs abundantly in the middle and upper parts of the Conglomeratic Limestones unit; in the lower part of this unit, their occurrence is seldom in both quarries. There is no evidence of any anoxic episode during the deposition of the Conglomeratic Limestones unit in Upper Silesia. The bright colour of the sediments, the occurrence of numerous conglomeratic layers (deposited by bottom and storm currents, see Chudzikiewicz, 1975; Hagdorn & Szulc, 2007) and especially an abundant and taxonomically diverse benthic fauna in both quarries (several bivalve species, gastropods, foraminifers, ostracods, occasional articulate brachiopods, echinoderm ossicles, feeding and habitable invertebrate-burrows) in the lower part of the Conglomeratic Limestones unit are advocating for a favourable oxygen level of benthic waters and bottom deposits during the time of sedimentary accumulation. Dwarfing of P. striatum bivalves then appears associated with populating a marine basin in time of gradual transition from hypersaline conditions (sabkha) environment (causing biotic collapse in primary bioproductivity) into normal conditions of salinity and bioproductivity. In such transient conditions, with a relatively high level of salinity, shallow depth and a low bioproductivity level, dwarfing of P. striatum could be a response to environmental stress. Sea-level changes are therefore considered as one of the possible causes of the Lilliput Effect due to the growing competition intensity between competitors for environmental resources, such as food and living space (e.g., Lockwood, 2005; Twitchett, 2006; Futuyma, 2008; Borths & Ausich, 2011). In case of sea regression, decreasing living space may lead to the disappearance of a species and even to its total extinction. Living organisms could therefore turn towards a decrease in average body size, in order to reduce their nutritional requirements and accommodate less space, both shortages supplying an advantage in the "arm race" for environmental resources (Borths & Ausich, 2011; Brom et al., 2015b).
Unfortunately, further investigation into other taxa response in such conditions has not been possible so far in the studied quarries. For instance, thin-shelled Entolium discites and foraminifers, frequently occurring in the lower part of the Conglomeratic Limestones unit, are found generally broken and their shell measurements are thus impossible. Meanwhile other taxa occur in relatively small quantity or their skeletons are disarticulated. Under normal salinity conditions, in not so deep parts of the marine basin (mid carbonate ramp, see Szulc, 1993), during the sedimentation of the 1st Wavy Limestones unit and the middle and upper parts of the Conglomeratic Limestones unit, P. striatum bivalves reached a "normal" shell size. Change in body size of P. striatum, population dwindling and/or blanking in time of unfavorable environmental conditions point out the fourth type of Lilliput Effect (decrease in body size with Lazarus gaps; Borths & Ausich, 2011). Small-sized organisms have a relatively higher chance of survival in a less-stable environment due to their lower metabolic requirement and earlier onset of sexual maturity (Borths & Ausich, 2011; Brom et al., 2015b). Moreover, organisms from oligotrophic environments react in a similar way by decreasing their body size since the probability of finding food availability is relatively lower (Brom et al., 2015a).
We would like to thank our anonymous reviewers for their valuable comments and suggestions of improvement on a preliminary version of this manuscript. The project has been partially financed from the funds of the Leading National Research Centre (KNOW) received by the Centre for Polar Studies for the period 2014-2018. The investigations were also supported by a grant of the University of Wrocław (1017/S/ING/15-IV/rn) and by NCN grant UMO-2015/17/N/ST10/03069.
Assmann P. (1937).- Revision der Fauna der Wirbellosen der oberschlesischen Trias. Abhandlungen der Preußischen Geologischen Landesanstalt.- Schweizerbart Science Publishers, Stuttgart, 133 p. [in German].
Benton M.J. (2009).- The Red Queen and the Court Jester: Species diversity and the role of biotic and abiotic factors through time.- Science, vol. 323, no. 5915, p. 728-732.
Bojkowski K. (1955).- Dolny wapień muszlowy w okolicy Szczakowej. Materiały do geologii obszaru śląsko-krakowskiego. Tom I.- Biuletyn Państwowego Instytutu Geologicznego, Warsaw, vol. 97, p. 229-258 [in Polish].
Borths M.R. & Ausich W.I. (2011).- Ordovician-Silurian Lilliput crinoids during the end-Ordovician biotic crisis.- Swiss Journal of Palaeontology, vol. 130, p. 7-18.
Brom K., Brachaniec T. & Salamon M.A. (2015a).- Troglomorphism in the middle Triassic crinoids from Poland.- The Science of Nature, Heidelberg-Berlin, vol. 102, no. 9-10, Article 60, 5 p.
Brom K.R., Salamon M.A., Ferré B., Brachaniec T. & Szopa K. (2015b).- The Lilliput effect in crinoids at the end of the Oceanic Anoxic Event 2: a case study from Poland.- Journal of Paleontology, vol. 89, no. 6, p. 1076-1081.
Chudzikiewicz L. (1975).- Intraformational conglomerates in the Gogolin Beds (Middle Triassic, southern Poland).- Annales de la Société Géologique de Pologne, Cracow, vol. 45, p. 3-20.
Cox L.R., Newell N.D., Boyd D. W., Branson C.C., Casey R., Chavan A., Coogan A.H., Dechaseaux C., Fleming C.A., Haas F., Hertlein L.G., Kauffman E.G., Keen A.M., Larocque A., Mcalester A.L., Moore R.C., Nuttall C.P., Perkins B.F., Puri H.S., Smith L.A., Soot-Ryen T., Stenzel H.B., Trueman E.R., Turner R.D. & Weir J. (1969).- Bivalvia. i-xxxviii + N1-N952. In: Moore R.C. (ed.), Treatise on invertebrate paleontology. Part N. Mollusca 6 (1-2).- Geological Society of America, Boulder and University of Kansas Press, Kansas, 952 p.
Fara E. (2001).- What are Lazarus taxa? In: History of biodiversity - Geological Journal, Special Issue, vol. 36, no. 3-4, p. 291-303.
Futuyma D.J. (2005).- Mechanizmy i drogi ewolucji. In: Futuyma D.J. (ed.), Ewolucja.- Wydawnictwo Uniwersytetu Warszawskiego, Warsaw, p. 43-65 [in Polish].
Gorzelak P., Salamon M.A. & Ferré B. (2011).- Pelagic crinoids (Roveacrinida, Crinoidea) discovered in the Neogene of Poland.- Naturwissenschaften, Heidelberg-Berlin, vol. 98, p. 903-908.
Hagdorn H. & Głuchowski E. (1993).- Palaeobiogeography and stratigraphy of Muschelkalk echinoderms (Crinoidea, Echinoidea) in Upper Silesia. In: Hagdron H. & Seilacher A. (eds.), Muschelkalk. Schöntaler Symposium 1991.- Goldschneck-Verlag, Stuttgart, Korb, p. 165-176.
Hagdorn H. & Szulc J. (2007).- Fieldtrip Guide. Stop I.1. Gogolin - inactive quarry. Stop II.3. Płaza - active quarry. Stop III.2. Żyglin - small active quarry. In: Szulc J. & Becker A. (eds.), International workshop on the Triassic of southern Poland.- Polish Geological Society, Polish Geological Institute, Institute of Geological Sciences, Jagiellonian University, Cracow, p. 46, 58 & 61.
Hallam A. (1997).- Mass extinctions and their aftermath.- Oxford University Press, Oxford, 328 p.
Hammer Ø., Ryan P. & Harper D. (2001).- PAST: Paleontological Statistics software package for education and data analysis.- Palaeontologia Electronica, vol. 4, p. 1-9.
Harries P.J. & Knorr P.O. (2009).- What does the 'Lilliput Effect' mean?- Palæogeography, Palæoclimatology, Palæoecology, vol. 284, p. 4-10.
Huang B., Harper D.A.T., Zhan R. & Rong J. (2010).- Can the Lilliput Effect be detected in the brachiopod faunas of South China following the terminal Ordovician mass extinction?- Palæogeography, Palæoclimatology, Palæoecology, vol. 285, p. 277-286.
Jablonski D. (1986).- Background and mass extinctions: the alternation of macroevolutionary regimes.- Science, vol. 231, no. 4734, p. 129-133.
Kowal-Linka M. (2010).- Origin of cone-in-cone calcite veins during calcitization of dolomites and their subsequent diagenesis: A case study from the Gogolin Formation (Middle Triassic), SW Poland.- Sedimentary Geology, vol. 224, p. 54-64.
Lockwood R. (2005).- Body size, extinction events, and the early Cenozoic record of veneroid bivalves: a new role for recoveries?- Paleobiology, vol. 31, p. 578-590.
McRoberts C.A. (2011).- Late Triassic Bivalvia (chiefly Halobiidae and Monotidae) from the Pardonet Formation, Williston Lake Area, Northeastern British Columbia, Canada.- Journal of Paleontology, vol. 85, p. 613-664.
Nawrocki J. & Szulc J. (2000).- The Middle Triassic magnetostratigraphy from the Peri-Tethys basin in Poland.- Earth and Planetary Science Letters, vol. 182, p. 77-92.
Niedźwiedzki R. (2002).- Revision of stratigraphic ranges of selected invertebrate taxa from the Muschelkalk in Silesia.- Geological Quarterly, Warsaw, vol. 46, p. 219-225.
Salamon M.A., Gorzelak P., Ferré B. & Lach R. (2010).- Roveacrinids (Crinoidea, Echinodermata) survived the Cretaceous-Paleogene (K-Pg) extinction event.- Geology, vol. 38, no. 10, p. 883-885.
Salamon M.A., Niedźwiedzki R., Gorzelak P., Lach R. & Surmik D. (2012a).- Bromalites from the Middle Triassic of Poland and the rise of the Mesozoic Marine Revolution.- Palæogeography, Palæoclimatology, Palæoecology, vol. 321-322, p. 142-150.
Salamon M.A., Niedźwiedzki R., Lach R., Brachaniec T. & Gorzelak P. (2012b).- Ophiuroids Discovered in the Middle Triassic Hypersaline Environment.- PLoS ONE, col. 7(11), e49798, doi:10.1371/journal.pone.0049798.
Senkowiczowa H. (1973).- Trias. In: Sokołowski S. (ed.), Budowa geologiczna Polski. Tom I. Stratygrafia. Część 2, Mezozoik.- Wydawnictwa Geologiczne, Warsaw, p. 15-140 [in Polish].
Szulc J. (1993).- Early Alpine tectonics and lithofacies succession in the Silesian part of the Muschelkalk basin. A synopsis. In: Hagdorn H. & Seilacher A. (eds.) Muschelkalk. Schöntaler Symposium 1991.- Goldschneck-Verlag, Stuttgart, Korb, p. 19-28.
Szulc J. (2000).- Middle Triassic evolution of the Northern peri-Tethys area as influenced by early opening of the Tethys Ocean.- Annales Societatis Geologorum Poloniae, Warsaw, vol. 70, p. 1-148.
Twitchett R.J. (2006).- The palaeoclimatology, palaeoecology and palaeoenvironmental analysis of mass extinction events.- Palæogeography, Palæoclimatology, Palæoecology, vol. 232, p. 190-213.
Urbanek A. (1993). Biotic crises in the history of Upper Silurian graptoloids: a palaeobiological model.- Historical Biology, London, vol. 7, p. 29-50.
Wade B.S. & Peterson P.N. (2008).- Planktonic foraminiferal turnover, diversity fluctuations and geochemical signals across the Eocene/Oligocene boundary in Tanzania.- Marine Micropaleontology, vol. 68, p. 244-255.
Wade B.S. & Twitchett R.J. (2009).- Extinction, dwarfing and the Lilliput effect. Palæogeography, Palæoclimatology, Palæoecology, vol. 284, p. 1-3.
Zawidzka K. (1975).- Conodont stratigraphy and sedimentary environment of the Muschelkalk in Upper Silesia.- Acta Geologica Polonica, vol. 25, p. 217-257.
Zatoń M., Salamon M.A., Boczarowski A & Sitek S. (2008).- Taphonomy of dense ophiuroid accumulations from the Middle Triassic of Poland.- Lethaia, vol. 41, p. 47-58.