◄ Carnets Geol. 16 (11) ►
Contents
[Introduction]
[Geological setting]
[Matherial and methods]
[Results]
[Systematic paleontology] [Discussion]
[Paleogeographic
remarks]
[Conclusions] and ... [Bibliographic references]
Babeş-Bolyai
University, Department of Geology and Center for Integrated Geological Studies,
str. M. Kogălniceanu nr. 1, 400084 Cluj-Napoca (Romania)
Babeş-Bolyai
University, Department of Geology and Center for Integrated Geological Studies,
str. M. Kogălniceanu nr. 1, 400084 Cluj-Napoca (Romania)
Published online in final form (pdf) on May 19, 2016
[Editor: Bruno Granier;
language editor: Robert W. Scott]
This study describes seventeen non-geniculate coralline algal species (orders Corallinales, Hapalidiales and Sporolithales) from the middle Miocene (lower-middle Badenian) red-algal limestones of the Transylvanian Basin, Gârbova de Sus Formation. For the description and identification at species level, we follow the common diagnostic features used for fossil species and some characters that are used as diagnostic for modern species (roof morphology for asexual conceptacles, the presence/absence of a layer of elongated cells below sporangial compartments and number of cells in paraphyses for Sporolithon, and measurements of gametangial and carposporangial conceptacles). Female conceptacles of Spongites fruticulosus Kützing are for the first time described in fossil material. We propose the attribution of Lithophyllum platticarpum Maslov to Spongites fruticulosus Kützing as a gametangial thallus with male conceptacles.
Central Paratethys; Badenian; rhodolith; gametangium; Spongites fruticulosus.
Chelaru R. & Bucur I.I. (2016).- The taxonomy of middle Miocene red algae from the Gârbova de Sus Formation (Transylvanian Basin, Romania).- Carnets Geol., Madrid, vol. 16, no. 11, p. 307-336.
Systématique des algues rouges du Miocčne moyen de la Formation Gârbova de Sus (Bassin Transylvanien, Roumanie).- Cette étude présente dix-sept espčces d'algues corallines non articulées (ordres des Corallinales, des Hapalidiales et des Sporolithales) provenant des calcaires ŕ algues rouges du Miocčne moyen (Badénien inférieur-moyen) de la Formation Gârbova de Sus du Bassin Transylvanien. Pour la description et l'identification au niveau spécifique, nous adoptons les caractéres diagnostiques habituellement utilisés chez les espčces fossiles et certaines caractéristiques qui sont considérées comme diagnostiques chez les espčces modernes (morphologie du toit pour les conceptacles asexués, la présence ou l'absence d'une couche de cellules allongées sous les compartiments sporangiaux et le nombre de cellules dans les paraphyses de Sporolithon, et les mesures des conceptacles gamétangiaux et carposporangiaux). Les conceptacles femelles de Spongites fruticulosus Kützing sont décrits pour la premičre fois chez des formes fossiles. Nous proposons le rattachement de Lithophyllum platticarpum Maslov ŕ Spongites fruticulosus Kützing pour un thalle gamétangial ŕ conceptacles mâles.
Paratéthys centrale ; Badénien ; rhodolithe ; gamétange ; Spongites fruticulosus.
The carbonate-siliciclastic succession of the Gârbova de Sus Formation cropping out at the western border of the Transylvanian Basin, mainly consists of coralline algae, larger benthic foraminifera and bryozoans. Although the importance of coralline algae as palaeoenvironmental indicators in Cenozoic carbonate deposits is very well documented elsewhere (among others: Fravega et al., 1994; Nebelsick & Bassi, 2000; Nebelsick et al., 2000; Rasser, 2000; Braga & Aguirre, 2001; Basso et al., 2008; Nebelsick et al., 2013; Coletti et al., 2015), the coralline algae from the Transylvanian Basin are still insufficiently described.
Some early contributions (Lörenthey, 1894a, 1894b; Koch, 1900; Pávay-Vajna, 1910; Şuraru, 1992) mention "Lithothamnium ramosissimum", a species considered representative of Middle Miocene deposits, within the so called "Lithothamniumkalke". Later Bucur & Filipescu (1994) studied the red algae from the western margin of the Transylvanian Basin and provided a detailed description and illustration of 13 species. A more recent study (Bucur & Filipescu, 2011) of the Lopadea Veche section presents more illustrations of coralline algae, but the identification of specimens remains at genus level.
New samples were collected for the present study; hence, this is not the re-examination of species previously identified by other authors. Some of the species described here are recorded for the first time in the Transylvanian Basin or the Central Paratethys.
The re-evaluation of species and genera of coralline algae from the Transylvanian Basin is needed in the light of the recent revisions of some type collections containing species described in the 19th and 20th centuries (Aguirre et al., 1996, 2011; Basso et al., 1998; Bassi et al., 2005, 2007; Vannucci et al., 2000, 2008; Iryu et al., 2012), and the introduction of new diagnostic features for the identification of fossil species (Hrabovský et al., 2015).
An important purpose of the present study is to identify features that are used in descriptions of modern red algae. Gametangial and carposporangial conceptacles are identified following Hrabovský et al. (2015). We focused on the description of specimens bearing these features, because of their rare identification or misidentification in fossil material as mastophoroid/lithophylloid corallines, exclusively on the basis of their uniporate conceptacles.
Based on the latter features, the present study provides a detailed taxonomic description of Middle Miocene (Badenian) red algae (Corallinales, Hapalidiales, Sporolithales) from the Lopadea Veche section, Transylvanian Basin. This work aims to contribute to a better understanding of the palaeogeographic distribution of red algal assemblages in the Middle Miocene Central Paratethys and to improve our knowledge of the anatomy of fossil red algae.
The Transylvanian Basin is an intra-Carpathian sedimentary basin, part of the Central Paratethys. The basin had a back-arc type evolution, under regional compressional stress (Ciulavu, 1999). On the western border, the Neogene sedimentary formations developed on the Mesozoic structures of the Trascău Mountains (Eastern Apuseni Mountains) consisting of ophiolites and Tithonian - lowermost Cretaceous limestones of the Bedeleu Nappe or olistoliths of Stramberk-like limestones of the Upper Cretaceous Râmeți Formation (Filipescu, 1996).
The Middle Miocene from this area is represented by the sedimentary deposits of the Câmpie Group. The algal-bioclastic limestones with marly-argillaceous intercalations are characteristic of the Badenian Gârbova de Sus Formation, cropping out between Arieș and Ampoi Valleys. The Gârbova de Sus Formation corresponds in the north-western part of the Transylvanian Basin to the Badenian Dej Formation represented by the Dej Tuff, which was dated at 14.38 Ma in the Ciceu-Giurgești area (Leeuw et al., 2013). The carbonate deposits formed in shallow-marine environments, along the Trascău Mountains, while deeper settings are characterized by the Dej Formation in the northern-central and eastern part of the Transylvanian Basin. The overlying Cheia Formation is represented by evaporites (salts and gypsum) that correspond to the earliest Late Badenian, Wielician stage (Hohenegger et al., 2014). They are overlain by marls with radiolarians and Spirialis of the Upper Badenian Pietroasa Formation.
One
of the most representative sections of Gârbova de Sus Formation is situated at
Lopadea Veche (Fig. 1.A-B ![]() ). The age of Gârbova de Sus Formation was initially
considered to be Early Badenian, but studies of the foraminiferal fauna from the
upper part of this section (Filipescu, 2001) extended it to the Wielician
stage, which, according to Hohenegger et al. (2014), represents the
earliest Late Badenian (earliest Serravallian). Other studies on this formation focused on the coral build-ups (Saint-Martin
et al., 2007) and green algae (Bucur
et al., 2011) from the Podeni section,
bryozoans from Gârbova de Sus section (Zágoršek et
al., 2010) or calcareous nannoplankton and foraminiferal assemblages, used
for biostratigraphic and paleoenvironmental interpretations (Hosu & Filipescu,
1995; Filipescu, 1996; Filipescu & Gîrbacea,
1997).
). The age of Gârbova de Sus Formation was initially
considered to be Early Badenian, but studies of the foraminiferal fauna from the
upper part of this section (Filipescu, 2001) extended it to the Wielician
stage, which, according to Hohenegger et al. (2014), represents the
earliest Late Badenian (earliest Serravallian). Other studies on this formation focused on the coral build-ups (Saint-Martin
et al., 2007) and green algae (Bucur
et al., 2011) from the Podeni section,
bryozoans from Gârbova de Sus section (Zágoršek et
al., 2010) or calcareous nannoplankton and foraminiferal assemblages, used
for biostratigraphic and paleoenvironmental interpretations (Hosu & Filipescu,
1995; Filipescu, 1996; Filipescu & Gîrbacea,
1997).
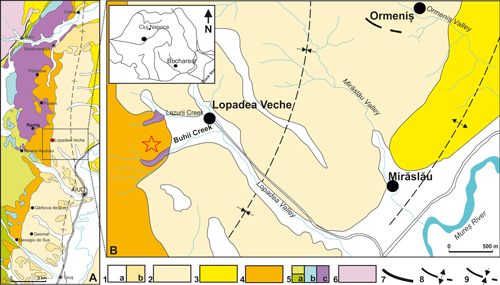
Click on thumbnail to enlarge the image.
Figure 1: Location of the studied area. A, Geological map with the western border of the Transylvanian Basin (modified after Lupu et al., 1967, and Săndulescu et al., 1978) showing the outcrops of Gârbova de Sus Formation; B, Detail of the Lopadea Veche area and location of the studied Lopadea Veche section on Buhii Valley; 1. Quaternary deposits (a, Holocene; b, Pleistocene); 2. Pannonian (Lopadea Formation); 3. Sarmatian (Măhăceni Formation); 4. Badenian (Gârbova de Sus Formation); 5. Mesozoic [(a, Cretaceous (Râmeți Formation); b, Jurassic (Bedeleu Nappe); c, Triassic-Jurassic volcanic arc)]; 6. Pre-Mesozoic basement units (metamorphites); 7. Thrust plane; 8. Anticline; 9. Syncline.
The
studied deposits of Lopadea Veche section are located in the Buhii Valley near
the Lopadea Veche village on the western border of the Transylvanian Basin (Fig.
1.B ![]() ). The succession consists of mixed
carbonate-siliciclastic deposits about 30m
thick that transgressively overlie the Mesozoic ophiolites of Trascău
Mountains starting with a basal microconglomerate.
). The succession consists of mixed
carbonate-siliciclastic deposits about 30m
thick that transgressively overlie the Mesozoic ophiolites of Trascău
Mountains starting with a basal microconglomerate.
The Lopadea Veche section was sampled at intervals of 10 up to 30 cm. Coralline algae were examined in a total of 218 thin sections of various dimensions (6x6 cm up to 12x12 cm), obtained from 156 samples, including 16 rhodoliths extracted from the upper part of the succession.
Algal growth-form terminology follows Woelkerling et al. (1993). We used the terminology proposed by Woelkerling (1988) for the description of the structure of thalli of coralline red algae and the descriptions of Johansen (1981). For species identification, accurate measurements were made using Image J-Software (Abrŕmoff et al., 2004) on photomicrographs made with Canon Powershot A640 mounted on a Zeiss microscope.
The abbreviations used in text and tables are the following: VC, ventral core of basal filaments; PF, peripheral filaments; M, mean value; SD, standard deviation; H1 and H2 for the internal heights in uniporate conceptacles and n for the number of measurements. The measured parameters of coralline algae cells and conceptacles follow Basso et al. (1996) and Rasser & Piller (1999). The measurements in the species descriptions belong either to a single specimen that shows as many features as possible or to multiple specimens identified as the same species. In tables, all dimensions are in µm.
The studied coralline algal assemblages from Lopadea Veche show that melobesioids are abundant and the most diversified, especially Lithothamnion species. Mastophoroids, represented by dominant specimens of Spongites fruticulosus, and sporolithoids are common. However, lithophylloids and peyssonelliaceans are rare and geniculate corallines are very rare.
The corallines are represented by fragments, crusts, free branches (mäerl - type) or rhodoliths and characterize different facies types. The association of unattached branches of Lithothamnion ramosissimum and Sporolithon sp. 1 indicates mäerl - type facies, while the rhodolithic levels show a greater diversification of species.
In addition to the described species, the studied samples comprise mastophoroid and melobesioid taxa of uncertain generic placement, Lithoporella melobesioides, Lithophyllum microsporum, Sporolithon lvovicum and Peyssonellia antiqua. The geniculate corallines are represented by undetermined specimens of Corallina and Jania, showing fragments of intergenicula lacking reproductive structures.
In this study we describe non-geniculate coralline algal species of the orders Corallinales, Hapalidiales and Sporolithales. The systematic positions of genus and higher taxonomic ranks of red algae belonging to the Corallinophycidae follow Woelkerling (1988), Braga et al. (1993), Harvey et al. (2003), and Kato et al. (2011) who separated three new subfamilies (Porolithoideae, Neogoniolithoideae, Hydrolithoideae) from Mastophoroideae. Considering the uncertain systematic position of Spongites (Kato et al., 2011), we follow M.D. Guiry & G.M. Guiry (2013) by taking into account the three new subfamilies (Kato et al., 2011) and assigning Spongites to Mastophoroideae (Hrabovský et al., 2015). The specimen showing uncertainties regarding the nature of conceptacles (asexual/ gametangial/ carposporangial) is noted ? Neogoniolithon, indicating the possibility of assignment to a gametangial thallus of Mesophyllum. When the shape of epithallial cells is unclear or not preserved, the melobesioids with non-coaxial ventral filaments are noted ? Lithothamnion.
Phylum Rhodophyta Wettstein, 1901
Class Florideophyceae Cronquist, 1960
Subclass Corallinophycidae Le Gall & Saunders, 2007
Order Corallinales Silva & Johansen, 1986
Family Corallinaceae Lamouroux, 1812 (Table 1)
Table 1: Comparison of biometric measurements of the identified species belonging to the family Corallinaceae.
| Species | Hydrolithon corculumis | Hydrolithon lemoinei | Spongites fruticulosus | Spongites sp. 1 | ? Neogoniolithon sp. | ||||||||||||||||
| n | Range | M | SD | n | Range | M | SD | n | Range | M | SD | n | Range | M | SD | n | Range | M | SD | ||
| VC cells | L | 7 | 13-22 | 16.3 | 3.3 | 20 | 15-21 | 17.8 | 1.9 | 20 | 10-19 | 12.8 | 2.8 | 20 | 10-19 | 14.1 | 1.9 | ||||
| D | 7 | 15-24 | 19.4 | 2.8 | 20 | 11-16 | 13.3 | 1.6 | 20 | 6-9 | 7.5 | 0.6 | 20 | 7-11 | 9.4 | 1.3 | |||||
| PF cells | L | 150 | 10-22 | 14.7 | 2.6 | 50 | 16-28 | 22.2 | 3.1 | 50 | 10-24 | 15.3 | 2.9 | 50 | 6-10 | 8.1 | 1 | 50 | 9-14 | 11.4 | 1.2 |
| L | 30 | 23-21 | 25.9 | 2.7 | |||||||||||||||||
| D | 150 | 6-14 | 10.8 | 1.2 | 50 | 10-19 | 14.3 | 2.6 | 50 | 8-16 | 11.7 | 1.6 | 50 | 5-10 | 6.8 | 1.1 | 50 | 5-8 | 6.6 | 0.7 | |
| Epithallial cells | L | 15 | 4-7 | 5 | 0.9 | 2 | 3-5 | ||||||||||||||
| D | 15 | 7-14 | 9.1 | 2 | 2 | 5-8 | |||||||||||||||
| Conceptacles | D | 11 | 185-311 | 262.7 | 41 | 2 | 163-250 | 33 | 240-484 | 387.2 | 55.6 | 22 | 363-715 | 541.6 | 116 | 9 | 552-642 | 603 | 33 | ||
| H | 12 | 93-129 | 111.5 | 11.9 | 2 | 113-220 | 33 | 151-213 | 182.9 | 16.4 | 22 | 115-248 | 178.7 | 33 | 9 | 284-496 | 371 | 63.6 | |||
| Pore canals | L | 3 | 48-74 | 1 | 167 | 16 | 86-154 | 110.3 | 20.6 | 10 | 70-252 | 139.1 | 69.5 | 1 | 230 | ||||||
| D | 3 | 25-40 | 1 | 37 | 16 | 63-120 | 94.5 | 17.4 | 10 | 86-126 | 104.7 | 16.8 | 1 | 64-87 | |||||||
Subfamily Hydrolithoideae Kato & Baba in Kato et al., 2011
Genus Hydrolithon (Foslie, 1905) Foslie, 1909
Type species. Hydrolithon reinboldii (Weber-van Bosse & Foslie) Foslie, 1909, Recent, Muras Reef, East Kalimantan, Indonesia.
Hydrolithon corculumis (Maslov, 1962) Braga et al., 2005
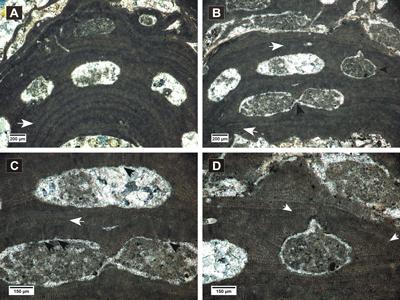
(Fig. 2.A-F ![]() )
)
Basionym. Lithophyllum corculumis Maslov, 1962, p. 80, Pl. 20, figs. 1-3.
Synonymy.
2005 Hydrolithon corculumis (Maslov) comb. nov.; Braga et al., p. 211, Fig. 2.f-h.
Stratigraphical range. Badenian.
Description.
Encrusting
thallus (Fig. 2.A-B ![]() ),
262-478 µm thick. Thallus shows dorsiventral dimerous organization (Fig.
2.C
),
262-478 µm thick. Thallus shows dorsiventral dimerous organization (Fig.
2.C ![]() ).
Cells of the ventral core (VC) are similar to those in the peripheral filaments
(PF) (Fig. 2.C
).
Cells of the ventral core (VC) are similar to those in the peripheral filaments
(PF) (Fig. 2.C ![]() ). The cells are squarish to rectangular,
10-22 µm long and 6-14
µm wide. The roof cells and the cells at the sides of conceptacles become more
elongated (Fig. 2.C-D & F
). The cells are squarish to rectangular,
10-22 µm long and 6-14
µm wide. The roof cells and the cells at the sides of conceptacles become more
elongated (Fig. 2.C-D & F ![]() ),
measuring 23-31 µm in length. Cell fusions are visible (Fig.
2.C-F
),
measuring 23-31 µm in length. Cell fusions are visible (Fig.
2.C-F ![]() ).
).
The uniporate conceptacles measure 185-311 µm in diameter and
93-129 in
height. The pore canals measure 48-74 µm in length and 25-40 µm in diameter
and are bordered by 3-4 cells perpendicular to the conceptacle roof (Fig.
2.C & E ![]() ).
).
Remarks. The investigated specimens fit the holotype description of Braga et al. (2005). Lithophyllum corculumis described and illustrated from the Badenian reefs of Poland (Pisera, 1985) shows higher conceptacles and the presence of cell fusions or secondary pit connections are unclear, suggesting that the specimen is not H. corculumis.
Studied thin sections. LVA 9, LVA 15, LVC 8x.
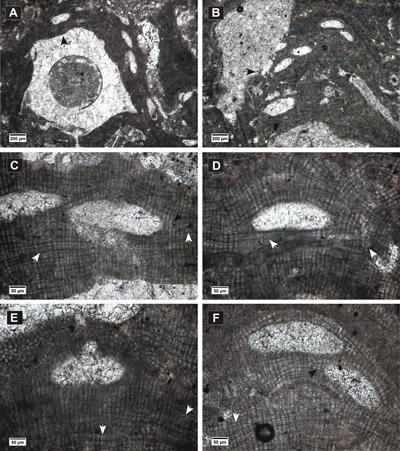
Click on thumbnail to enlarge the image.
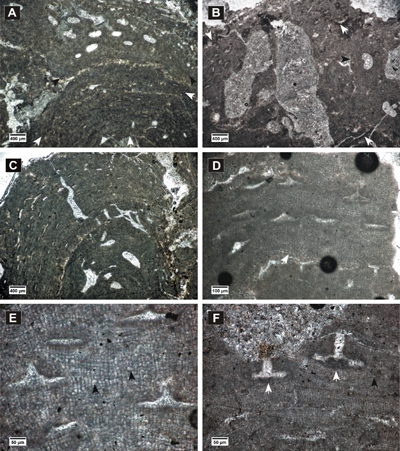
Figure 2: Hydrolithon corculumis. A-B, encrusting thalli showing uniporate conceptacles; C-F, peripheral filaments with cell fusions (white arrows) and cells elongated at the sides of the conceptacles and above them (black arrows); in C, E, slightly conical pore canals are visible.
Hydrolithon lemoinei (Miranda, 1935) Aguirre et al., 2011
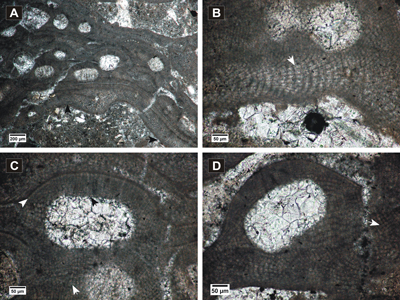
(Fig. 3.A-D ![]() )
)
Basionym. Melobesia lemoinei Miranda, 1935, p. 284-285, Fig. 3A, B, Pl. 38, fig. 1.
Synonymy.
2011 Hydrolithon lemoinei (Miranda) comb. nov.; Aguirre et al., p. 282, Figs. 5F & 6A-D.
2015 Hydrolithon lemoinei (Miranda) Aguirre et al., 2011; Hrabovský et al., p. 15-17, Fig. 16A-B.
Stratigraphical range. Oligocene - Badenian.
Description. Encrusting thallus (Fig.
3.A ![]() ),
90-384 µm thick. Thallus shows dorsiventral dimerous organization
(Fig. 3.B
),
90-384 µm thick. Thallus shows dorsiventral dimerous organization
(Fig. 3.B ![]() ). Cells of the VC are
13-22 µm long and 15-24 µm wide. Cells in the PF are predominantly rectangular,
16-28
µm long and 10-19
µm wide and do not show a
particular alignment. The presence of cell fusions is noticeable in both VC and
PF (Fig. 3.B
). Cells of the VC are
13-22 µm long and 15-24 µm wide. Cells in the PF are predominantly rectangular,
16-28
µm long and 10-19
µm wide and do not show a
particular alignment. The presence of cell fusions is noticeable in both VC and
PF (Fig. 3.B ![]() ).
).
The conceptacles are uniporate and protruding at the thallus surface
(Fig. 3.C-D ![]() ). They have
flat floors and measure 163-250 µm in diameter and 113-220 µm
in height. The pore canal is
cylindrical, 167 µm long and 37 µm wide. The cells surrounding
the pore canal are more or less perpendicular to the conceptacle roof (Fig.
3.C-D
). They have
flat floors and measure 163-250 µm in diameter and 113-220 µm
in height. The pore canal is
cylindrical, 167 µm long and 37 µm wide. The cells surrounding
the pore canal are more or less perpendicular to the conceptacle roof (Fig.
3.C-D ![]() ).
).
Remarks. The specimen fits the description of Aguirre et al. (2011) for the type material.
Studied thin section. LVC 3.
Click on thumbnail to enlarge the image.
Figure 3: Hydrolithon lemoinei. A, encrusting dimerous thallus (arrows) showing uniporate conceptacles; B, dimerous thallus with VC cells similar to PF cells, cell fusions; C-D, uniporate conceptacles with flat floors showing roof cells more or less parallel with the pore canal (arrows).
Subfamily Mastophoroideae Setchell, 1943
Genus Spongites Kützing, 1841
Type species. Spongites fruticulosus Kützing, 1841, Recent, Mediterranean Sea.
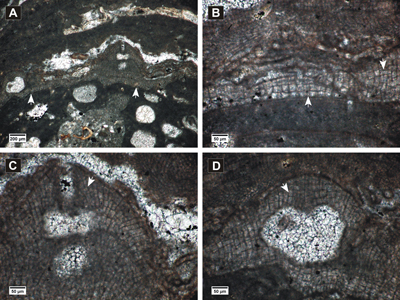
Spongites fruticulosus Kützing, 1841
(Fig. 4.A-D ![]() - 5.A-F
- 5.A-F ![]() )
)
Synonymy.
1993 Spongites albanensis (Lemoine) comb. nov.; Braga et al., p. 544, Pl. 2, figs. 1, 3-4.
2006 Spongites fruticulosus Kützing; Basso & Rodondi, p. 404, Figs. 1-34.
Stratigraphical range. Oligocene - Recent.
Description. Encrusting and protuberant
thalli (Fig. 4.A ![]() ) with monomerous dorsiventral organization.
Non-coaxial VC
(Fig. 4.B
) with monomerous dorsiventral organization.
Non-coaxial VC
(Fig. 4.B ![]() ) consists of cells
15-21
µm long and 11-16
µm wide. Cells in
PF vary in shape and their arrangement is irregular; however, weak horizontal
rows can be observed. PF cells measure 10-24
µm in length and 8-16
µm in diameter.
Epithallial cells are flattened or rounded (Fig. 4.C
) consists of cells
15-21
µm long and 11-16
µm wide. Cells in
PF vary in shape and their arrangement is irregular; however, weak horizontal
rows can be observed. PF cells measure 10-24
µm in length and 8-16
µm in diameter.
Epithallial cells are flattened or rounded (Fig. 4.C ![]() ),
4-7
µm long and 7-14
µm wide. Cell
fusions are frequent in PF and VC (Fig. 4.B & D
),
4-7
µm long and 7-14
µm wide. Cell
fusions are frequent in PF and VC (Fig. 4.B & D ![]() ).
).
Asexual conceptacles are buried in thalli and measure 240-484
µm in diameter and 151-213 µm in height. The pore canals are 86-154
µm long and 63-120 µm wide at the base. The cells lining the pore
canal are oriented more or less parallel to the roof (Fig. 4.C-D ![]() ).
).
Dioecious gametangial thalli are observed in fusion with the thallus
bearing asexual conceptacles (Fig. 5.A-B ![]() ), or encrusting other species (Fig.
5.C
), or encrusting other species (Fig.
5.C ![]() ). Growth forms and vegetative structures are consistent with the thallus in
asexual phase. VC is monomerous non-coaxial (Fig. 5.D
). Growth forms and vegetative structures are consistent with the thallus in
asexual phase. VC is monomerous non-coaxial (Fig. 5.D ![]() ), with cells
10-22 µm
in length and 7-14 µm in diameter and PF cells measure 8-18
µm in length and 5-11 µm in diameter. Multiple cell
fusions are observable (Fig. 5.E
), with cells
10-22 µm
in length and 7-14 µm in diameter and PF cells measure 8-18
µm in length and 5-11 µm in diameter. Multiple cell
fusions are observable (Fig. 5.E ![]() ).
).
The small male conceptacles (Fig. 5.A-E ![]() ) measure
137-202
µm in diameter and 32-58 µm in height. Pore canals are 27-106
µm long and 30-66
µm wide at the
base.
) measure
137-202
µm in diameter and 32-58 µm in height. Pore canals are 27-106
µm long and 30-66
µm wide at the
base.
Two female conceptacles are observed slightly protruding at the thallus
surface (Fig. 5.F ![]() ). Organic remains of carpogonial branches are observed in the
conceptacle floors (Fig. 5.F
). Organic remains of carpogonial branches are observed in the
conceptacle floors (Fig. 5.F ![]() ). The chambers measure
122-171 µm
in diameter and 42-55 µm in height, with pore canals 65-117 µm long and 36-45
µm wide.
). The chambers measure
122-171 µm
in diameter and 42-55 µm in height, with pore canals 65-117 µm long and 36-45
µm wide.
Remarks. The
identified gametangial specimens correspond to the ones collected from the
Mediterranean and described by Basso & Rodondi
(2006).
Gametangial thalli were identified in multiple thin sections, not only in fusion
with the thalli bearing asexual conceptacles, but also encrusting other species
in rhodoliths (Fig.
5.C ![]() ) or as free nodules; nevertheless, they were always
present in thin sections containing thalli in asexual phase. A close affinity of
the identified gametangial male thalli with Lithophyllum platticarpum Maslov is observed
(Table 2). The
type material of L. platticarpum was
redescribed by Braga et al.
(2005) and they concluded that L.
platticarpum is the gametangial thallus of a mastophoroid species. Moreover,
Spongites albanensis (Lemoine) Braga,
Bosence & Steneck, a heterotypic synonym of Spongites
fruticulosus Kützing
(Braga et al., 2009), has been identified in association with L. platticarpum in Miocene rhodoliths from NW Italy (Vannucci
et al., 1994). Taking all the above in
consideration, we consider that Lithophyllum
platticarpum Maslov, 1962, should be assigned to Spongites fruticulosus Kützing,
as
a gametangial thallus with male conceptacles.
) or as free nodules; nevertheless, they were always
present in thin sections containing thalli in asexual phase. A close affinity of
the identified gametangial male thalli with Lithophyllum platticarpum Maslov is observed
(Table 2). The
type material of L. platticarpum was
redescribed by Braga et al.
(2005) and they concluded that L.
platticarpum is the gametangial thallus of a mastophoroid species. Moreover,
Spongites albanensis (Lemoine) Braga,
Bosence & Steneck, a heterotypic synonym of Spongites
fruticulosus Kützing
(Braga et al., 2009), has been identified in association with L. platticarpum in Miocene rhodoliths from NW Italy (Vannucci
et al., 1994). Taking all the above in
consideration, we consider that Lithophyllum
platticarpum Maslov, 1962, should be assigned to Spongites fruticulosus Kützing,
as
a gametangial thallus with male conceptacles.
Table 2: Biometric measurements of the fossil gametangial thalli of S. fruticulosus Kützing (1 this study) in comparison with the biometry of recent specimens from the Mediterranean (2 Basso & Rodondi, 2006) and fossil specimens of L. platticarpum Maslov described from Ukraine (3 Braga et al., 2005) and Poland (4 Studencki, 1989) and Leptolithophyllum platticarpum (Maslov) Poignant from Corsica (5 Orszag-Sperber & Poignant, 1977).
| Species | Spongites
fruticulosus gametangial thallus |
Spongites
fruticulosus gametangial thallus |
Lithophyllum platticarpum | Lithophyllum platticarpum | Lepto. platticarpum | ||||
| Locality | (Romania)1 | (Mediterranean)2 | (Ukraine)3 | (Poland)4 | (Corsica)5 | ||||
| n | Range | M | SD | Range | Range | Range | Range | ||
| VC cells | L | 50 | 10-22 | 14 | 2.5 | 10-32 | 15-30 | 20 | |
| D | 50 | 7-14 | 10 | 1.5 | 5-17 | 10-14 | 10 | ||
| PF cells | D | 100 | 8-18 | 13 | 2.1 | 6-28 | 10-20 | 10-15 | 10-15 |
| D | 100 | 5-11 | 8 | 1.2 | 5-22 | 10-15 | 9-12 | 10 | |
| Male conceptacles | D | 30 | 137-202 | 170 | 18.6 | 190-230 | 210-230 | 120-150 | 200 |
| H | 30 | 32-58 | 41 | 7.5 | 40-70 | 35-50 | 30-40 | 40 | |
| Pore canals | L | 23 | 27-106 | 54 | 21.3 | 70-100 | 15 | ||
| D | 23 | 30-66 | 45 | 10 | 40-60 | ||||
| Female conceptacles | D | 2 | 122-171 | 125-345 | |||||
| H | 2 | 42-55 | 170-308 (with pore canal) |
||||||
| Pore canals | L | 2 | 65-117 | ||||||
| D | 2 | 36-45 | |||||||
Studied thin sections. The measurements for the thallus bearing tetrasporangial conceptacles were made for a specimen in LVA 11, where the features can be observed more clearly. Male thalli were studied in LVA 9, LVA 10 and LVB 6 and the female thallus was identified in LVB 5.
Click on thumbnail to enlarge the image.
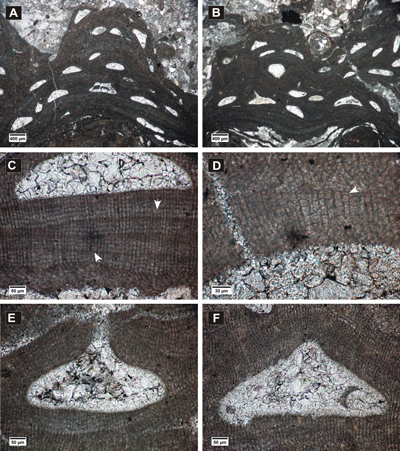
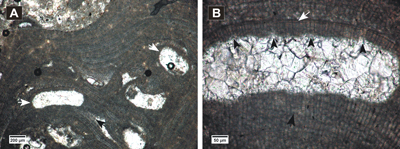
Figure 4: Spongites fruticulosus. A, thick encrusting thallus showing abundant uniporate conceptacles; B, non-coaxial ventral filaments, cell fusions present in both VC and PF (arrows); C-D, uniporate conceptacles with cells lining the pore canals more or less parallel to the roof (black arrow); C, layer of rounded and flattened epithallial cells (arrows); D, PF with multiple cell fusions (white arrow), uniporate conceptacle with slightly raised columella (black arrow).
Click on thumbnail to enlarge the image.
Figure 5: Spongites fruticulosus, gametangial thalli. A-B, encrusting male thallus (white arrows) fused with thallus bearing asexual conceptacles (black arrows); C, encrusting male thallus (arrow) bearing abundant conceptacles; D, male thallus showing non-coaxial VC (arrow); E, detail of male conceptacles, peripheral filaments with cell fusions (arrows); F, female thallus with peripheral filaments showing cell fusions (black arrow) and uniporate conceptacles protruding at the thallus surface, with conceptacle floors showing organic remains of carpogonial branches (white arrows).
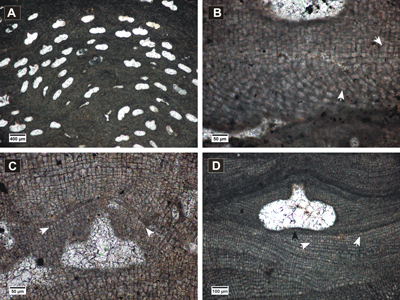
Spongites sp. 1
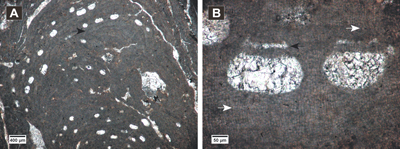
(Fig. 6.A-F ![]() )
)
Stratigraphical range. Late Eocene - Badenian.
Description.
Encrusting thallus forming warty protuberances in the fertile parts (Fig.
6.A-B ![]() ), with monomerous dorsiventral organization. VC appears
non-coaxial
(Fig. 6.C
), with monomerous dorsiventral organization. VC appears
non-coaxial
(Fig. 6.C ![]() ),
60-110 µm thick and consists of cells 10-19 µm long and 6-9 µm wide. PF
zonated, with bands 78-150 µm thick. PF cells measure 6-10 µm in length and 5-10 µm in diameter. The epithallial cells seem flattened (Fig.
6.D
),
60-110 µm thick and consists of cells 10-19 µm long and 6-9 µm wide. PF
zonated, with bands 78-150 µm thick. PF cells measure 6-10 µm in length and 5-10 µm in diameter. The epithallial cells seem flattened (Fig.
6.D ![]() ),
3-5 µm
long and 5-8 µm wide. Cell fusions are common in PF (Fig. 6.C
),
3-5 µm
long and 5-8 µm wide. Cell fusions are common in PF (Fig. 6.C ![]() ).
).
Uniporate
conceptacles (Fig. 6.A-B & E-F ![]() ) have mostly flat floors, rounded sides and measure
363-715 µm in diameter and 115-248 µm in height. The pore canals are 70-252 µm
long and their diameter at the base is 86-126 µm. They are lined by 1-14
cells.
) have mostly flat floors, rounded sides and measure
363-715 µm in diameter and 115-248 µm in height. The pore canals are 70-252 µm
long and their diameter at the base is 86-126 µm. They are lined by 1-14
cells.
Remarks. The growth form and biometric measurements of the studied specimen are similar to the ones of ? Lithophyllum perrandoi Airoldi described by Vannucci et al. (2008); however, because of the presence of cell fusions in PF and the absence of evident secondary pit connections, we decided to attribute it to Spongites.
Studied thin section. LV 56.
Click on thumbnail to enlarge the image.
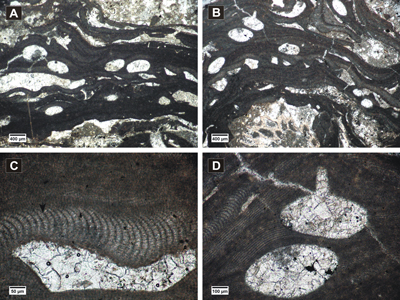
Figure 6: Spongites sp. 1. A-B, encrusting and protuberant thalli showing uniporate conceptacles; C, section of thallus showing thin non-coaxial VC (black arrow) and zoned PF with secondary pit connections (white arrows); D, flattened epithallial cells (arrow); E-F, detail of uniporate conceptacles.
Subfamily Neogoniolithoideae Kato & Baba in Kato et al., 2011
Genus Neogoniolithon Setchell & Manson, 1943
Type species. Neogoniolithon fosliei (Heydrich) Setchell & Manson, 1943, Recent, El Tor, Sinai Peninsula, Egypt.
? Neogoniolithon sp. 1
(Fig. 7.A-D ![]() )
)
Description. Encrusting thallus, with
dorsiventral organization and monomerous construction (Fig.
7.A-B ![]() ). VC coaxial
(Fig. 7.C
). VC coaxial
(Fig. 7.C ![]() ),
100-180 µm thick with cells 10-19
µm long and 7-11
µm wide. PF cells
measure 9-14 µm in length and 5-8
µm in diameter. A layer of
mostly rounded epithallial cells is observed (Fig. 7.C
),
100-180 µm thick with cells 10-19
µm long and 7-11
µm wide. PF cells
measure 9-14 µm in length and 5-8
µm in diameter. A layer of
mostly rounded epithallial cells is observed (Fig. 7.C ![]() ). Cell fusions are
frequent in VC and PF (Fig. 7.C
). Cell fusions are
frequent in VC and PF (Fig. 7.C ![]() ).
).
Large uniporate conceptacles have rounded sides, mostly concave upward
floors and are protruding at the thallus surface, mound-like (Fig.
7.A-B & D ![]() ) They
measure 552-642 µm in diameter and 284-496
µm in height. Pore canals are
conical (Fig. 7.D
) They
measure 552-642 µm in diameter and 284-496
µm in height. Pore canals are
conical (Fig. 7.D ![]() ) and measure 230 µm
in height and in diameter, 87 µm at the base to 64 µm at the top. Roof cells are similar to
the PF cells. The cells lining the pore canals are perpendicular to the
conceptacle roof.
) and measure 230 µm
in height and in diameter, 87 µm at the base to 64 µm at the top. Roof cells are similar to
the PF cells. The cells lining the pore canals are perpendicular to the
conceptacle roof.
Remarks. The specimen shows similarities with Lithophyllum bassanense Mastrorilli (Mastrorilli, 1973; Pisera & Studencki, 1989). The coaxial VC shows evident cell fusions, therefore the specimen cannot be assigned to Lithophyllum. The presence of coaxial VC, cell fusions and uniporate conceptacles indicate Neogoniolithon species, but the uniporate conceptacles can also be gamentangial/ carposporangial; thereby, this specimen can be assigned to Neogoniolithon or to Mesophyllum, as a gametangial or carposporangial thallus. The studied sample includes also encrusting thalli of M. cf. incisum (description below), that resembles this specimen in biometric measurements and the protruding, mound-like nature of conceptacles. However, the gametangial and carposporangial thallus of M. incisum do not show similarities with this specimen; therefore we decided to attribute it to ? Neogoniolithon.
Further investigations and reassessment of the Lithophyllum bassanense Mastrorilli holotype may indicate a certain taxonomic position of this specimen.
Studied thin section. LV 39.
Click on thumbnail to enlarge the image.
Figure 7: ? Neogoniolithon sp.1. A-B, encrusting fertile thalli showing uniporate conceptacles protruding at the thallus surface; C, coaxial ventral filaments, cell fusions present (arrow), layer of epithallial cells (arrowhead); D, detail of uniporate conceptacle with cylindrical-conical pore canal, regular arrangement of PF cells observed above the conceptacles.
Order Hapalidiales Nelson, Sutherland, Farr & Yoon in Nelson et al., 2015
Family Hapalidiaceae (Gray, 1864) Harvey et al., 2003
Subfamily Melobesioideae Bizzozero, 1885
Genus Lithothamnion Heydrich, 1897 (Table 3)
Type species. Lithothamnion muelleri Lenormand ex Rosanoff, 1866, Recent, Western Port Bay, Victoria.
Table 3: Comparison of biometric measurements of the identified Lithothamnion species.
| Species | Lithothamnion crispatum |
Lithothamnion ramosissimum |
Lithothamnion roveretoi |
Lithothamnion sp. 1 |
|||||||||||||
| n | Range | M | SD | n | Range | M | SD | n | Range | M | SD | n | Range | M | SD | ||
| VC cells | L | 15 | 13-17 | 14.4 | 1.3 | 25 | 10-16 | 12.8 | 1.6 | 7 | 12-17 | 14.5 | 1.6 | ||||
| D | 15 | 9-11 | 10.2 | 0.8 | 25 | 8-12 | 9.6 | 1.1 | 7 | 6-10 | 7.6 | 1.1 | |||||
| PF cells | L | 50 | 11-17 | 13.8 | 1.3 | 50 | 9-21 | 13.9 | 3.6 | 25 | 9-16 | 12.1 | 1.8 | 25 | 20-30 | 25.6 | 2.5 |
| L | 50 | 18-27 | 22.9 | 3.0 | 25 | 13-18 | 15 | 1.4 | |||||||||
| D | 50 | 8-13 | 10.2 | 1.2 | 50 | 8-12 | 9.5 | 1.1 | 25 | 7-10 | 8.6 | 0.7 | 25 | 8-11 | 9.7 | 0.7 | |
| Epithallial cells | L | 10 | 2-5 | 3.7 | 0.9 | 25 | 5-7 | 6.3 | 0.6 | ||||||||
| D | 10 | 7-11 | 8.9 | 1.1 | 25 | 6-12 | 8.8 | 1.3 | |||||||||
| Conceptacles | D | 4 | 375-512 | 463.4 | 60.9 | 17 | 240-535 | 389.9 | 82.6 | 2 | 412-477 | 1 | 630 | ||||
| H | 4 | 167-201 | 184.1 | 15.2 | 17 | 98-148 | 116.7 | 15.3 | 2 | 120-159 | 1 | 240 | |||||
| Roof thickness | 22-46 | 43-48 | 40-55 | 52-66 | |||||||||||||
| Nr. of roof cells | 2-4 | 3-4 | 4-6 | 4-6 | |||||||||||||
| Roof cells | L | 15 | 6-11 | 8.3 | 1.2 | 25 | 7-12 | 9.1 | 1.4 | 25 | 9-13 | 11.3 | 1.3 | ||||
| D | 15 | 5-8 | 6.3 | 0.9 | 25 | 5-8 | 6.6 | 0.8 | 25 | 7-11 | 8.5 | 1.2 | |||||
| Pore canals | L | 2 | 20-24 | ||||||||||||||
| D | 2 | 30-32 | |||||||||||||||
| Species | Lithothamnion sp. 2 |
Lithothamnion sp. 3 |
? Lithothamnion sp. 4 |
||||||||||
| n | Range | M | SD | n | Range | M | SD | n | Range | M | SD | ||
| VC cells | L | 8 | 8-11 | 9.5 | 1.0 | 25 | 8-11 | 9.7 | 0.9 | 6 | 9-14 | 11.6 | 2.0 |
| D | 8 | 6-8 | 6.9 | 0.6 | 25 | 7-10 | 9.1 | 0.8 | 7 | 8-11 | 9.1 | 1.1 | |
| PF cells | L | 25 | 7-13 | 9.1 | 1.7 | 30 | 7-14 | 10.3 | 1.8 | 50 | 9-15 | 12 | 1.6 |
| L | 25 | 14-26 | 20.1 | 2.8 | |||||||||
| D | 25 | 6-8 | 6.9 | 0.6 | 30 | 6-9 | 7.2 | 1.0 | 50 | 7-10 | 8.8 | 0.8 | |
| Epithallial cells | L | 7 | 3-5 | 3.4 | 1.0 | ||||||||
| D | 7 | 5-8 | 5.9 | 1.1 | |||||||||
| Conceptacles | D | 7 | 252-373 | 288.4 | 44.2 | 16 | 404-858 | 612.4 | 125.9 | 6 | 475-707 | 585.3 | 81.2 |
| H | 7 | 133-165 | 148.2 | 12.1 | 16 | 208-356 | 295.8 | 47 | 7 | 289-384 | 331.6 | 35.1 | |
| Roof thickness | 36-55 | 78-100 | 122-137 | ||||||||||
| Nr. of roof cells | 3-4 | 6-9 | 6-9 | ||||||||||
| Roof cells | L | 25 | 6-9 | 7.5 | 0.7 | 10 | 7-9 | 7.8 | 1.0 | 25 | 9-14 | 11.5 | 1.2 |
| D | 25 | 5-7 | 5.5 | 0.5 | 10 | 5-7 | 6.4 | 0.7 | 25 | 5-7 | 6.1 | 0.5 | |
| Pore canals | L | 20-46 | 8 | 13-42 | 22.1 | 9.3 | |||||||
| D | 14-16 | 8 | 13-43 | 22.4 | 9.2 | ||||||||
Lithothamnion crispatum Hauck, 1878
(Fig. 8.A-D ![]() )
)
Synonymy.
2011 Lithothamnion crispatum Hauck; Basso et al., p. 145, Figs. 1-30, tab. 1.
2016 Lithothamnion crispatum Hauck; Coletti et al., p. 30-31, Figs. 1-8, tab. 2.
Stratigraphical range. Burdigalian - Recent.
Description. Encrusting and protuberant thallus showing a
branch up to 5 mm thick (Fig. 8.A ![]() ). Thallus monomerous, with dorsiventral
organization. VC non-coaxial (Fig. 8.B
). Thallus monomerous, with dorsiventral
organization. VC non-coaxial (Fig. 8.B ![]() ),
125-158 µm
thick, with cells 13-17 µm long and 9-11 µm wide. Zonation in PF is visible.
Zones are 5-7 cells in maximum thickness. PF cells measure 11-17 µm in length
and 8-13 µm in diameter. Epithallial cells are flattened and flared (Fig.
8.CD
),
125-158 µm
thick, with cells 13-17 µm long and 9-11 µm wide. Zonation in PF is visible.
Zones are 5-7 cells in maximum thickness. PF cells measure 11-17 µm in length
and 8-13 µm in diameter. Epithallial cells are flattened and flared (Fig.
8.CD ![]() ),
2-5 µm in length and 7-11 µm in diameter. Meristematic cells are as long as or
longer than those subtending them. Cell fusions are observed in VC and PF (Fig.
8.B-C
),
2-5 µm in length and 7-11 µm in diameter. Meristematic cells are as long as or
longer than those subtending them. Cell fusions are observed in VC and PF (Fig.
8.B-C ![]() ).
).
Tetrasporangial
conceptacles have rounded sides and flat floors, 375-512 µm in diameter and 167-201 µm in height. The conceptacle roofs are
22-46 µm thick and consist of 2-4 cell layers. Where some depressions are visible (Fig. 8.D ![]() ), most probably
connected with the pore canals, the roofs are thinner. The roof cells are 6-11
µm in length and 5-8 µm in diameter, smaller than other PF cells. Two wedged-shaped cells are observed lining a pore canal (Fig. 8.D
), most probably
connected with the pore canals, the roofs are thinner. The roof cells are 6-11
µm in length and 5-8 µm in diameter, smaller than other PF cells. Two wedged-shaped cells are observed lining a pore canal (Fig. 8.D ![]() ).
).
Remarks. The depressions and wedge-shaped cells in the asexual conceptacle roofs, branched growth form, vegetative anatomy, and biometric measurements of the identified specimen are similar to the ones redescribed by Basso et al. (2011) from the Lithothamnion crispatum Hauck lectotype and the ones described by Coletti et al. (2016) from fossil specimens.
Studied thin section. LV 49.
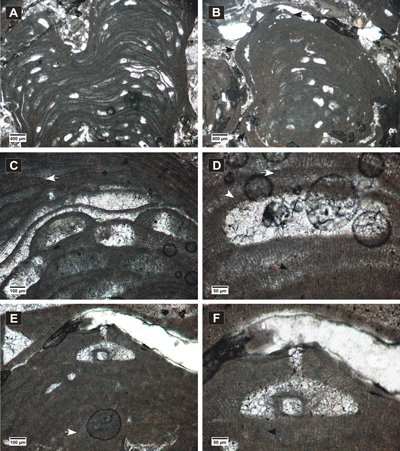
Click on thumbnail to enlarge the image.
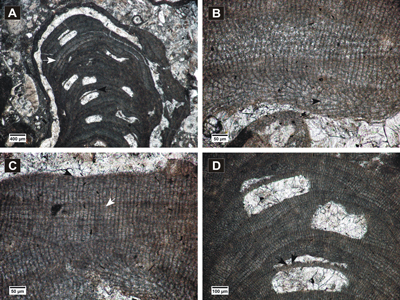
Figure 8: Lithothamnion crispatum. A, protuberant thallus showing zonation in PF (white arrow) and concavities above the conceptacle roofs (black arrow); B, cell fusion (arrow) in non-coaxial VC; C, cell fusion (white arrow) in PF, flared epithallial cell (black arrow); D, multiporate conceptacle showing depressions in the roof and wedge-shaped cells lining the pore canal (arrows).
Lithothamnion ramosissimum (Reuss, 1847) Piller, 1994
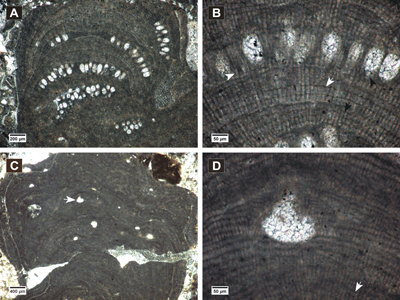
(Fig. 9.A-D ![]() )
)
Basionym. Nullipora ramosissima Reuss, 1847.
Synonymy.
1946 Palaeothamnium archaeotypum nov. sp.; Conti, p. 42-46, Pl. III, fig. 3a-c; Pl. VIII, fig. 1a-c.
1996 Lithothamnion ramosissimum (Reuss); Aguirre et al., p. 3-8, Pl. I, figs. 1-6; Pl. II, figs. 1-6.
Stratigraphical range. Middle Miocene.
Description. Encrusting and fruticose thallus, with
protuberances up to 3 mm in diameter and 7 mm in length (Fig.
9.A-B ![]() ). Thallus
monomerous with dorsiventral organization. VC non-coaxial (Fig.
9.C
). Thallus
monomerous with dorsiventral organization. VC non-coaxial (Fig.
9.C ![]() ), around 180
µm thick and consists of cells 10-16 µm long and 8-12
µm wide. PF shows zonation in
protuberances (Fig. 9.A-B
), around 180
µm thick and consists of cells 10-16 µm long and 8-12
µm wide. PF shows zonation in
protuberances (Fig. 9.A-B ![]() ), zones extending from 70 µm to a maximum of 180 µm
in thickness. PF cells measure 9-21 µm in length and 8-12 µm in diameter.
Epithallial cells are not observed. Cell fusions are present in both VC and PF (Fig.
9.C
), zones extending from 70 µm to a maximum of 180 µm
in thickness. PF cells measure 9-21 µm in length and 8-12 µm in diameter.
Epithallial cells are not observed. Cell fusions are present in both VC and PF (Fig.
9.C ![]() ).
).
Multiporate conceptacles measure 240-535
µm in diameter and 98-148 µm in height. Almost all the chambers show early
stages of development, being filled with large elongated cells (Fig.
9.D ![]() ). Below
the conceptacle floor is observed a layer of elongated cells (Fig.
9.D
). Below
the conceptacle floor is observed a layer of elongated cells (Fig.
9.D ![]() ),
18-27 µm
in length, similar to the cells from the sides of the chambers and smaller than
those forming the chambers. The roofs are 43-48 µm thick, composed of 3-4
cells.
),
18-27 µm
in length, similar to the cells from the sides of the chambers and smaller than
those forming the chambers. The roofs are 43-48 µm thick, composed of 3-4
cells.
Remarks. Aguirre et al. (1996) described Lithothamnion rammosissimum (Reuss) from the type localities and Basso et al. (1997) redescribed the lectotype material. The growth form, vegetative anatomy and biometric measurements of the identified specimen fit their descriptions.
Although the conceptacle heights of this species do not exceed 120 µm in the lectotype material (Conti, 1946; Basso et al., 1997), we take into consideration the measurements of specimens studied from type localities (Aguirre et al., 1996) as well.
Studied thin section. LV 2.
Click on thumbnail to enlarge the image.
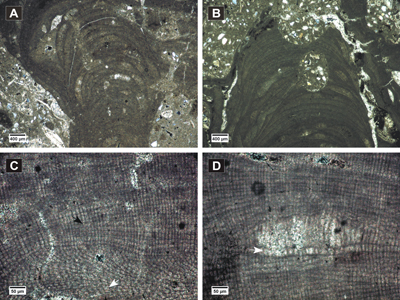
Figure 9: Lithothamnium ramosissimum. A-B, fertile fruticose protuberances showing zonation in peripheral filaments; C, non-coaxial ventral filaments showing cell fusions (white arrow), cell fusion in PF (black arrow); D, detail of multiporate conceptacle with layer of elongated cells (white arrow) and filling with longer cells (black arrow).
Lithothamnion roveretoi Airoldi, 1932
(Fig. 10.A-B ![]() )
)
Synonymy.
2010 Lithothamnion roveretoi Airoldi; Vannucci et al., p. 224-226, Pl. 1, figs. 1-8, tab. 1.
2015 Lithothamnion roveretoi Airoldi; Hrabovský et al., p. 6-7, Fig. 5A-D.
Stratigraphical range. Middle-Late Eocene - Badenian.
Description.
Growth
form encrusting and slightly protuberant (Fig. 10.A ![]() ). Thallus monomerous, with
dorsiventral organization, 560-600 µm thick. VC is non-coaxial (Fig.
10.A-B
). Thallus monomerous, with
dorsiventral organization, 560-600 µm thick. VC is non-coaxial (Fig.
10.A-B ![]() )
and 56-90 µm thick, with cells measuring 12-17 µm in length by 6-10 µm in
diameter. PF slightly zoned, 312-496 µm thick with cells 9-16 µm in length and
7-10 µm in diameter. Cell fusion present in both ventral and peripheral
filaments (Fig. 10.B
)
and 56-90 µm thick, with cells measuring 12-17 µm in length by 6-10 µm in
diameter. PF slightly zoned, 312-496 µm thick with cells 9-16 µm in length and
7-10 µm in diameter. Cell fusion present in both ventral and peripheral
filaments (Fig. 10.B ![]() ). Flattened cells, presumed epithallial, are observed in
conceptacle roofs (Fig. 10.B
). Flattened cells, presumed epithallial, are observed in
conceptacle roofs (Fig. 10.B ![]() ).
).
The conceptacles have flattened roofs and floors and were raised about 61-77 µm at the thallus surface. The chambers measure 412-477 µm in diameter and 120-159 µm in height. The roofs are 40-55 µm thick and composed of 4-6 cell layers. Roof cells measure 7-12 µm in length and 5-8 µm in diameter, conformable with the surrounding PF cells.
Remarks. The specimen fits the description of Vannucci et al. (2010) for the type material of Lithothamnion roveretoi Airoldi; however, the conceptacles of the identified specimen are slightly higher. The occurrence of this species in the Upper Eocene of Romania (Bucur et al., 1987) is uncertain because it has different biometric measurements and no illustrations are provided. Therefore, we suggest that Lithothamnion roveretoi appeared in the Central Paratethys only during the middle Miocene.
Studied thin section. LV 7x.

Click on thumbnail to enlarge the image.
Figure 10: Lithothamnion roveretoi. A, encrusting and superposed thalli, non-coaxial VC (white arrow); B, cell fusions present in the peripheral filaments (white arrow), flattened and flared epithallial cell (black arrow).
Lithothamnion sp. 1
(Fig. 11.A-B ![]() )
)
Description. Unattached branched thallus (Fig.
11.A ![]() ), with VC unrecognizable. PF zoned,
600-660 µm
thick, with elongated cells 20-30 µm in length and 8-11 in diameter. The common
cells are shorter, measuring 13-18 in length. Cell fusions frequent in PF (Fig.
11.B
), with VC unrecognizable. PF zoned,
600-660 µm
thick, with elongated cells 20-30 µm in length and 8-11 in diameter. The common
cells are shorter, measuring 13-18 in length. Cell fusions frequent in PF (Fig.
11.B ![]() ). Epithallial cells are flattened
and flared (Fig. 11.B
). Epithallial cells are flattened
and flared (Fig. 11.B ![]() ),
5-7 µm in length and 6-12 µm in diameter.
),
5-7 µm in length and 6-12 µm in diameter.
Only one conceptacle could be
adequately measured, 630 µm in diameter and 240 µm in height, with roof and
floor relatively flattened and rounded corners. The roof is 52-66 µm thick,
formed by 4-6 cells, with peripheral rim. The roof cells are 9-13 µm in length
and 7-11 µm in diameter, smaller than the surrounding PF cells. Pore canals
seem conical (Fig. 11.B ![]() ),
20-24 µm long and the basal part 30-32 µm wide.
),
20-24 µm long and the basal part 30-32 µm wide.
Studied thin section. LV 60.
Click on thumbnail to enlarge the image.
Figure 11: Lithothamnion sp. 1. A, encrusting thallus showing multiporate conceptacles (white arrows) and elongated cells in PF (black arrow); B, detail of a multiporate conceptacle, layer of flattened and flared epithallial cells (white arrow), cell fusion in PF (black arrow), conical pore canals (black arrowheads).
Lithothamnion sp. 2
(Fig. 12.A-F ![]() )
)
Description. Unattached branched thalli (Fig.
12.A-B ![]() ), up to 8
mm in length and 5 mm in width. Ventral filaments are observed only above the
conceptacles (Fig. 12.D
), up to 8
mm in length and 5 mm in width. Ventral filaments are observed only above the
conceptacles (Fig. 12.D ![]() ). VC cells measure
8-11
µm in length and 6-8 µm in diameter. PF is irregularly zoned (Fig. 12.C
). VC cells measure
8-11
µm in length and 6-8 µm in diameter. PF is irregularly zoned (Fig. 12.C ![]() ),
2-3 or 5-6 cells long.
PF cells are 7-13 µm long and 6-8
µm wide. Epithallial cells are
flattened and flared (Fig. 12.D
),
2-3 or 5-6 cells long.
PF cells are 7-13 µm long and 6-8
µm wide. Epithallial cells are
flattened and flared (Fig. 12.D ![]() ). Cell fusions are observed
(Fig. 12.D
). Cell fusions are observed
(Fig. 12.D ![]() ).
).
Asexual conceptacles were mostly raised above the thallus surface and
then become buried due to continuous growing (Fig. 12.C ![]() ). The chambers measure
252-373 µm in diameter and 133-165
µm in height and they commonly
appear filled with large cells (Fig. 12.A-C
). The chambers measure
252-373 µm in diameter and 133-165
µm in height and they commonly
appear filled with large cells (Fig. 12.A-C ![]() ). The roof is
36-55 µm
thick, 3-4 cells long. The roof cells are 6-9 µm long and 5-7 µm wide, similar
to the surrounding PF cells.
). The roof is
36-55 µm
thick, 3-4 cells long. The roof cells are 6-9 µm long and 5-7 µm wide, similar
to the surrounding PF cells.
Presumed gametangial thallus is observed encrusting the thallus in
asexual phase. VC is non-coaxial (Fig. 12.E ![]() ), with cells
8-10 µm
(M = 8.9; SD = 0.7) long and 4-8 µm (M = 6.1; SD = 1.2) wide (n = 20). PF is zoned with cells
7-11
µm
(M = 8.8; SD = 0.6) long and 5-8 µm (M = 6.3; SD = 0.7) wide (n = 50). VC and
PF cells are similar to the cells in the tetrasporangial thallus. Cell fusions
are present (Fig. 12.F
), with cells
8-10 µm
(M = 8.9; SD = 0.7) long and 4-8 µm (M = 6.1; SD = 1.2) wide (n = 20). PF is zoned with cells
7-11
µm
(M = 8.8; SD = 0.6) long and 5-8 µm (M = 6.3; SD = 0.7) wide (n = 50). VC and
PF cells are similar to the cells in the tetrasporangial thallus. Cell fusions
are present (Fig. 12.F ![]() ). Presumed gametangial conceptacles are observed slightly
raised above the thallus (Fig. 12.B & E-F
). Presumed gametangial conceptacles are observed slightly
raised above the thallus (Fig. 12.B & E-F ![]() ). The chamber with the visible pore
canal measures 378 µm in diameter and 315 µm (H1) / 92 µm (H2)
in height. The pore canal is 126 µm long and 41 µm wide.
). The chamber with the visible pore
canal measures 378 µm in diameter and 315 µm (H1) / 92 µm (H2)
in height. The pore canal is 126 µm long and 41 µm wide.
Studied thin section. LV 74.
Click on thumbnail to enlarge the image.
Figure 12: Lithothamnion sp. 2. A, fertile lumpy protuberance; B, gametangial thallus at the surface of a protuberance (arrows point to gametangial conceptacles); C, peripheral filaments showing irregular zonation (arrow) and multiporate conceptacles protruding and buried in thallus; D, detail of multiporate conceptacle with ventral filaments above the roof (white arrow), cell fusion (black arrow), layer of flattened and flared epithallial cells (white arrowhead); E, gametangial thallus showing uniporate conceptacle slightly raised at the thallus surface, non-coaxial ventral filaments (arrow); F, detail of gametangial conceptacle, cell fusion (arrow).
Lithothamnion sp. 3
(Fig. 13.A-F ![]() )
)
Description. Encrusting thallus with warty protuberances, up
to 7 mm long and 5 mm wide. VC is non-coaxial (Fig. 13.B ![]() ),
390-430 µm
thick and consists of squarish to rectangular cells measuring 8-11 µm in length
and 7-10 µm in diameter. PF irregularly zoned with cells 7-14 µm long and 6-9
µm wide. The epithallial cells are flat and flared (Fig. 13.C
),
390-430 µm
thick and consists of squarish to rectangular cells measuring 8-11 µm in length
and 7-10 µm in diameter. PF irregularly zoned with cells 7-14 µm long and 6-9
µm wide. The epithallial cells are flat and flared (Fig. 13.C ![]() ) and measure
3-5
µm in length and 5-8 µm in diameter.
) and measure
3-5
µm in length and 5-8 µm in diameter.
Multiporate
conceptacles are abundant, slightly raised and have flattened or slightly sunken
pore plates (Fig. 13.A & C-D ![]() ). The chambers have rounded sides and measure
404-858 µm in diameter and 208-356 µm in height. The conceptacle roofs are 78-100 µm thick,
6-9 cells long. Roof cells measure 7-9 µm in length and 5-7
µm in diameter, similar to the PF cells. The pore canals are 20-46 µm long and
14-16 µm wide at the base.
). The chambers have rounded sides and measure
404-858 µm in diameter and 208-356 µm in height. The conceptacle roofs are 78-100 µm thick,
6-9 cells long. Roof cells measure 7-9 µm in length and 5-7
µm in diameter, similar to the PF cells. The pore canals are 20-46 µm long and
14-16 µm wide at the base.
Two
conceptacles, clearly uniporate and presumed gametangial, are observed in
thallus apparently fused with the thallus bearing asexual conceptacles (Fig.
13.E-F ![]() ). The chambers measure
520-660 µm in diameter, 370 µm (H1)
and 195 µm (H2) in height, with pore canal 145 µm long and 77 µm
wide.
). The chambers measure
520-660 µm in diameter, 370 µm (H1)
and 195 µm (H2) in height, with pore canal 145 µm long and 77 µm
wide.
Studied thin section. LV 11.
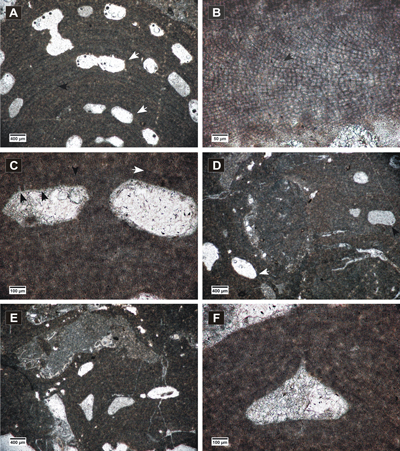
Click on thumbnail to enlarge the image.
Figure 13: Lithothamnion sp. 3. A, encrusting protuberant thallus; B, non-coaxial ventral filaments with cell fusions (arrow); C, detail of multiporate conceptacles showing pore canals (black arrows), slightly sunken pore plate (white arrow), layer of flattened and flared epithallial cells (black arrowhead); D, presumed gametangial thallus (white arrow points to uniporate conceptacle) in fusion with the specimen in asexual phase (black arrow points to multiporate conceptacle); E. presumed gametangial thallus; F, detail of the uniporate conceptacle.
? Lithothamnion sp. 4
(Fig. 14.A-D ![]() )
)
Description. Encrusting and slightly
protuberant thallus (Fig. 14.A-B ![]() ). VC is
50-60 µm
thick, non-coaxial, observed mainly above conceptacles (Fig.
14.B-C
). VC is
50-60 µm
thick, non-coaxial, observed mainly above conceptacles (Fig.
14.B-C ![]() ). Thallus monomerous with dorsiventral
organization. Cells in VC measure 9-14 µm in length and 8-11
µm in diameter. PF zoned,
showing bands 105-130 µm thick composed of 4-6 cells. PF
cells are mostly rectangular, 9-15 µm in length and 7-10 µm in diameter. Elongated
cells are present by the conceptacle sides, 14-26 µm long. Epithallial cells are
hardly distinguished and their shape is uncertain, as well as the size of
meristeme initials in comparison with cells subtending them. Cell fusion
present.
). Thallus monomerous with dorsiventral
organization. Cells in VC measure 9-14 µm in length and 8-11
µm in diameter. PF zoned,
showing bands 105-130 µm thick composed of 4-6 cells. PF
cells are mostly rectangular, 9-15 µm in length and 7-10 µm in diameter. Elongated
cells are present by the conceptacle sides, 14-26 µm long. Epithallial cells are
hardly distinguished and their shape is uncertain, as well as the size of
meristeme initials in comparison with cells subtending them. Cell fusion
present.
Asexual conceptacles were raised above the thallus surface and then buried. The chambers have rounded sides, slightly convex-up floors and measure 475-707 µm in diameter and 289-384 µm in height. The roof is 122-137 µm thick and consists of filaments 6-9 cells long. Roof cells are 9-14 long and 5-7 µm wide, narrower than other PF cells. Pore canals are conical, 13-43 µm wide at the base and 13-42 µm long.
Presumed carposporongial conceptacle is
found buried in the same thallus (Fig. 14.B & D ![]() ).
It measures 450 µm in diameter and 350 µm in height. The pore is 110 µm long
and has a conical shape, 97 µm in diameter at the base and 35 µm in the top.
).
It measures 450 µm in diameter and 350 µm in height. The pore is 110 µm long
and has a conical shape, 97 µm in diameter at the base and 35 µm in the top.
Studied thin section. LV 5.
Click on thumbnail to enlarge the image.
Figure 14: ? Lithothamnion sp. 4. A, encrusting, slightly protuberant thallus showing zonation in peripheral filaments (arrow); B, superposed thalli, two adjacent multiporate conceptacles (black arrow), non-coaxial ventral filaments (white arrows), presumed carposporangial conceptacle (black arrowhead); C, multiporate conceptacles with conical pore canals (black arrows), non-coaxial ventral filaments (white arrow); D, detail of presumed carposporangial conceptacle, cell fusions (white arrows).
Genus Mesophyllum Lemoine, 1928 (Table 4)
Type species. Mesophyllum lichenoides Lemoine, 1928, Recent, Cornwall, England.
Table 4: Comparison of biometric measurements of the identified Mesophyllum and Phymatolithon species.
| Species | Mesophyllum sp. 1 | Mesophyllum sp. 2 | Phymatolithon calcareum | ||||||||||
| n | Range | M | SD | n | Range | M | SD | n | Range | M | SD | ||
| VC cells | L | 20 | 14-23 | 17 | 2.1 | 25 | 19-26 | 21.7 | 1.9 | 20 | 12-18 | 14.9 | 1.4 |
| D | 20 | 11-15 | 13.1 | 1.3 | 25 | 5-12 | 8 | 1.9 | 20 | 7-12 | 9.7 | 1.1 | |
| PF cells | L | 20 | 9-13 | 11.4 | 1.4 | 50 | 9-17 | 11.9 | 1.7 | 50 | 6-13 | 9.4 | 1.6 |
| D | 20 | 6-9 | 7.6 | 0.8 | 50 | 6-9 | 7.8 | 0.8 | 50 | 5-8 | 6.3 | 0.7 | |
| Conceptacles | D | 10 | 193-387 | 290.2 | 47.9 | 4 | 253-353 | 319.3 | 46.9 | 19 | 154-300 | 218.8 | 37.3 |
| H | 10 | 159-274 | 216.5 | 35.7 | 4 | 223-305 | 259.7 | 40.4 | 19 | 89-160 | 128.7 | 19.8 | |
| Roof thickness | 65-95 | 54-96 | 38-47 | ||||||||||
| Nr. of roof cells | 6-7 | 5-7 | 3-5 | ||||||||||
| Roof cells | L | 20 | 5-10 | 7.6 | 0.8 | 20 | 5-9 | 7.6 | 1.3 | 15 | 6-8 | 6.8 | 0.4 |
| D | 20 | 5-7 | 5.9 | 0.4 | 20 | 4-7 | 5.5 | 1.0 | 15 | 5-6 | 5.7 | 0.3 | |
| Pore canals | L | 2 | 52-61 | ||||||||||
| D | 2 | 7-12 | |||||||||||
Mesophyllum sp. 1
(Fig. 15.A-D ![]() )
)
Description.
Encrusting monomerous thallus with dorsiventral organization, usually superposed
and forming short protuberances (Fig. 15.A ![]() ). Coaxial to
non-coaxial VC
(Fig. 15.A-B
). Coaxial to
non-coaxial VC
(Fig. 15.A-B ![]() ), up to 218 µm thick. VC
cells measure 14-23 µm in length and 11-15 µm in diameter. PF cells are 9-13
µm long and 6-9 µm wide. Epithallial cells are rounded (Fig.
15.C
), up to 218 µm thick. VC
cells measure 14-23 µm in length and 11-15 µm in diameter. PF cells are 9-13
µm long and 6-9 µm wide. Epithallial cells are rounded (Fig.
15.C ![]() ) and the
meristeme initials are as long as or longer than cells subtending them. Cell
fusions are present (Fig. 15.B-D
) and the
meristeme initials are as long as or longer than cells subtending them. Cell
fusions are present (Fig. 15.B-D ![]() ).
).
Multiporate conceptacles are protruding at the thallus surface and then
become buried in thallus. The chambers have rounded sides and flattened to
concave floors (Fig. 15.A & C-D ![]() ) and measure
193-387 µm in diameter and 159-274
µm in height. The roofs are convex, 65-95 µm thick and 6-7 celled, each cell 5-10 µm long and
5-7 µm wide. The roof cells are mostly rounded and smaller
than the surrounding PF cells. A hardly observable narrower cell is observed
lining a pore canal (Fig. 15.C
) and measure
193-387 µm in diameter and 159-274
µm in height. The roofs are convex, 65-95 µm thick and 6-7 celled, each cell 5-10 µm long and
5-7 µm wide. The roof cells are mostly rounded and smaller
than the surrounding PF cells. A hardly observable narrower cell is observed
lining a pore canal (Fig. 15.C ![]() ).
).
Studied thin sections. LV 19, LV 51.
Click on thumbnail to enlarge the image.
Figure 15: Mesophyllum sp.1. A, encrusting protuberant thalli with non-coaxial to coaxial ventral filaments (arrows); B, coaxial ventral filaments with cell fusions (arrow); C-D, multiporate conceptacles and peripheral filaments showing cell fusions (white arrows); C, layer of rounded epithallial cells (white arrowhead), narrow cell at the base of the pore canal (black arrow).
Mesophyllum sp. 2
(Fig. 16.A-D ![]() )
)
Description.
Encrusting
monomerous thallus with dorsiventral organization (Fig. 16.A ![]() ). Coaxial VC
(Fig. 16.A-B
). Coaxial VC
(Fig. 16.A-B ![]() ),
180-200 µm thick, consists of cells 18-26 µm long and 5-12
µm wide. Weak zonation is
hardly observed in PF consisting of cells 9-17
µm long and 6-9 µm wide. Epithallial cells are
flattened to round (Fig. 16.C
),
180-200 µm thick, consists of cells 18-26 µm long and 5-12
µm wide. Weak zonation is
hardly observed in PF consisting of cells 9-17
µm long and 6-9 µm wide. Epithallial cells are
flattened to round (Fig. 16.C ![]() ). Cell fusions are frequent in both VC and PF
(Fig. 16.B & D
). Cell fusions are frequent in both VC and PF
(Fig. 16.B & D ![]() ).
).
Multiporate conceptacles have different
shapes, from elliptical to round or even trapezoidal (Fig.
16.A-B ![]() ). The chambers
become buried by the ventral filaments when thalli are superposed (Fig.
16.A
). The chambers
become buried by the ventral filaments when thalli are superposed (Fig.
16.A ![]() ). They
measure 253-353 µm in diameter and 223-305 µm in height. The roofs have sunken
pore plates and raised peripheral rim (Fig. 16.C
). They
measure 253-353 µm in diameter and 223-305 µm in height. The roofs have sunken
pore plates and raised peripheral rim (Fig. 16.C ![]() ),
54-96 µm thick and 5-7 cells
long. Roof cells measure 5-9 µm in length and 4-7 µm in diameter, smaller than
the surrounding PF cells. The pore canals are long (Fig. 16.C
),
54-96 µm thick and 5-7 cells
long. Roof cells measure 5-9 µm in length and 4-7 µm in diameter, smaller than
the surrounding PF cells. The pore canals are long (Fig. 16.C ![]() ),
52-61 µm in length and 7-12 µm wide. The cells lining the pore canals
seem similar to the rest of roof cells.
),
52-61 µm in length and 7-12 µm wide. The cells lining the pore canals
seem similar to the rest of roof cells.
Remarks. The trapezoidal shape of the conceptacles and cell measurements of this specimen show similarities with M. curtum Lemoine redescribed by Aguirre & Braga (1998), but the conceptacles are larger and the pore plates are sunken. The biometric measurements of this specimen does not fit any description of known species with sunken pore plates.
Studied thin section. LV 48.

Click on thumbnail to enlarge the image.
Figure 16: Mesophyllum sp. 2. A, encrusting thalli with coaxial VC (black arrows) and multiporate conceptacles; B, cell fusion in ventral filaments (white arrow), trapezoidal multiporate conceptacles with long pore canals and sunken pore plates (black arrows); C, detail of multiporate conceptacle showing clearly a sunken pore plate and rounded epithallial cell (arrow), cells lining the pore canals similar to the other roof cells; D, layer of flattened to round epithallial cells (black arrow), cell fusions in PF (white arrows).
Genus Phymatolithon Foslie, 1898 (Table 4)
Type species. Phymatolithon calcareum (Pallas, 1766) Adey & McKibbin, 1970, Recent, Falmouth Harbour, England.
Phymatolithon calcareum (Pallas, 1766) Adey & McKibbin, 1970
(Fig. 17.A-B ![]() )
)
Basionym. Millepora calcarea Pallas, 1766.
Synonymy.
1986 Phymatolithon calcareum (Pallas) Adey & McKibbin; Woelkerling & Irvine, Figs. 1-15.
1994 Phymatolithon calcareum (Pallas) Adey & McKibbin; Basso, p. 579, Pl. 1, figs. 1-4; Pl. 3, figs. 1-4.
Stratigraphical range. Oligocene - Recent.
Description. Fruticose
thallus (Fig. 17.A ![]() ) showing a hardly distinguishable
non-coaxial VC
(Fig.
17.B
) showing a hardly distinguishable
non-coaxial VC
(Fig.
17.B ![]() ).
The cells in core filaments are 12-18 µm long and 7-12 µm wide. Zonation of
the PF is observable (Fig. 17.A
).
The cells in core filaments are 12-18 µm long and 7-12 µm wide. Zonation of
the PF is observable (Fig. 17.A ![]() ); each zone is made of
7-9 squarish to
rectangular cells. Cells in PF are 6-13 µm in length and 5-8 µm in diameter.
Hardly noticeable epithallial cells seem rounded. Cell fusions are present (Fig.
17.B
); each zone is made of
7-9 squarish to
rectangular cells. Cells in PF are 6-13 µm in length and 5-8 µm in diameter.
Hardly noticeable epithallial cells seem rounded. Cell fusions are present (Fig.
17.B ![]() ).
).
Tetrasporangial
conceptacles are abundant (Fig. 17.A ![]() ) and have
rounded sides and relatively flat roofs and floors. They measure 154-300
µm in diameter and 89-160 µm
in height. The roofs are 38-47 µm thick and
made of 3-5 small cell layers. The roof cells are 6-8 µm
long and 5-6 µm wide. Distinct rims with a
straight to concave shape are observed above the conceptacle roofs. (Fig.
17.B
) and have
rounded sides and relatively flat roofs and floors. They measure 154-300
µm in diameter and 89-160 µm
in height. The roofs are 38-47 µm thick and
made of 3-5 small cell layers. The roof cells are 6-8 µm
long and 5-6 µm wide. Distinct rims with a
straight to concave shape are observed above the conceptacle roofs. (Fig.
17.B ![]() ).
).
Remarks. The specimen fits the descriptions of Basso (1994, 1997) for fossil and living species.
Studied thin section. LVC 9X.
Click on thumbnail to enlarge the image.
Figure 17: Phymatolithon calcareum. A, Protuberant fertile thalli showing weak zonation (arrow); B, multiporate conceptacles with calcified concavities (black arrow), ventral filaments above the chambers (upper white arrow), cell fusion (lower white arrow).
Order Sporolithales Le Gall, Payri, Bittner & Saunders, 2009
Family Sporolithaceae Verheij, 1993
Subfamily Sporolithoideae Setchell, 1943
Genus Sporolithon Heydrich, 1897 (Table 5)
Type species. Sporolithon ptychoides Heydrich, 1897, Recent, Sinai Peninsula, Egypt.
Table 5: Biometric measurements of the identified Sporolithon species.
| Species | Sporolithon praeerythraeum | Sporolithon sp. 1 | |||||||||||||||
| sporangial thallus |
gametangial thallus |
sporangial thallus |
gametangial thallus |
||||||||||||||
| n | Range | M | SD | n | Range | M | SD | n | Range | M | SD | n | Range | M | SD | ||
| VC cells | L | 20 | 12-20 | 16.9 | 2.4 | 15 | 14-20 | 16.8 | 1.8 | ||||||||
| D | 20 | 9-13 | 10.7 | 1.1 | 15 | 8-12 | 10 | 0.8 | |||||||||
| PF cells | L | 50 | 12-27 | 20.6 | 3.3 | 50 | 14-30 | 20.2 | 4.1 | 50 | 11-16 | 13.2 | 1.2 | 50 | 13-19 | 16.6 | 1.5 |
| L | 25 | 18-25 | 21.5 | 1.9 | |||||||||||||
| D | 50 | 7-10 | 8.6 | 0.8 | 50 | 7-12 | 9.3 | 1.2 | 50 | 8-11 | 9.5 | 0.7 | 50 | 8-12 | 9.7 | 0.8 | |
| Sporangial compartments | D | 16 | 45-78 | 58.1 | 8.4 | 52 | 41-67 | 54.2 | 5.3 | ||||||||
| H | 16 | 88-118 | 103.6 | 7.5 | 52 | 102-158 | 129.8 | 11.8 | |||||||||
| Elongated cells below sp. comp. | L | 7 | 25-31 | 27.3 | 2.1 | ||||||||||||
| L | 7 | 7-9 | 7.9 | 0.9 | |||||||||||||
| Nr. of cells in paraphyses | 3-6 | 3-6 | |||||||||||||||
| Longest cells in paraphyses | L | 10 | 22-30 | 26.7 | 2.2 | ||||||||||||
| D | 10 | 5-8 | 6.8 | 0.7 | |||||||||||||
| Male conceptacles | D | 2 | 210-240 | ||||||||||||||
| H | 2 | 39-44 | |||||||||||||||
| Female conceptacles | D | 2 | 224-232 | ||||||||||||||
| H | 2 | 61-79 | |||||||||||||||
| Carposporangial conceptacles | D | 1 | 164 | ||||||||||||||
| H | 1 | 190 | |||||||||||||||
Sporolithon praeerythraeum (Airoldi, 1932) Vannucci et al., 2000
(Fig. 18.A-D ![]() )
)
Basionym. Archaeolithothamnium praeerithraeum Airoldi, 1932.
Synonymy.
2000 Sporolithon praeerythraeum (Airoldi, 1932) comb. nov.; Vannucci et al., p. 193-197, Fig. 2, Pls. 1-2, tab. 2.
Stratigraphical range. Late Eocene - Badenian.
Description.
Encrusting lumpy thallus with VC not visible (Fig. 18.A ![]() ). The PF
shows horizontal layers of rectangular to more elongated cells (Fig.
18.B
). The PF
shows horizontal layers of rectangular to more elongated cells (Fig.
18.B ![]() ). PF
cells measure 12-27 µm
in length and 7-10 µm
in diameter. Both cell fusions and secondary pit connections are present (Fig.
18.B
). PF
cells measure 12-27 µm
in length and 7-10 µm
in diameter. Both cell fusions and secondary pit connections are present (Fig.
18.B ![]() ).
).
Sporangial compartments are buried
in thallus and measure 88-118
µm
in length and 45-78 µm
in diameter. They are separated by 1-3 filaments in paraphyses, 3-6 cells long.
A layer of elongated cells is present below the compartments (Fig.
18.B ![]() ). These
cells represent the longest cells in paraphyses and measure 25-31 µm
in length and 7-9 µm in diameter. Stalk cells are rounded to trapezoidal (Fig.
18.B
). These
cells represent the longest cells in paraphyses and measure 25-31 µm
in length and 7-9 µm in diameter. Stalk cells are rounded to trapezoidal (Fig.
18.B ![]() )
)
The presumed carposporangial thallus (Fig.
18.C ![]() ) is represented by a
lumpy protuberance. The VC is not observable. PF cells are 14-30
µm long and 7-12
µm
wide. Cell fusions and secondary pit connections are present (Fig.
18.D
) is represented by a
lumpy protuberance. The VC is not observable. PF cells are 14-30
µm long and 7-12
µm
wide. Cell fusions and secondary pit connections are present (Fig.
18.D ![]() ). Within
the thallus are observed a few structures resembling carposporangial
conceptacles. The pear-shaped chamber showing a pore canal (Fig.
18.D
). Within
the thallus are observed a few structures resembling carposporangial
conceptacles. The pear-shaped chamber showing a pore canal (Fig.
18.D ![]() ) is 164 µm
in diameter and 190 µm
in height.
) is 164 µm
in diameter and 190 µm
in height.
Remarks. Even though the ventral filaments are not observed, the presence of the presumed carposporangial thallus in the same sample with the specimen bearing sporangial compartments confirms the assignment to S. praeerythraeum, due to the resemblance of both thalli with the redescribed holotype (Vannucci et al., 2000).
Studied thin section. LV 110.
Click on thumbnail to enlarge the image.
Figure 18: Sporolithon praeerythraeum. A, encrusting, lumpy thallus; B, detail of peripheral filaments showing cell fusion (white arrow) and secondary pit connection (black arrow), sporangial compartments form on elongated cells (white arrowhead), stalk cell (black arrowhead); C, presumed carposporangial thallus; D, detail of peripheral filaments showing the same cell arrangement with the thallus in asexual phase, presumed carposporangial conceptacle, cell fusion (white arrow), secondary pit connection (black arrow).
Sporolithon sp. 1
(Figs. 19.A-F ![]() - 20.A-D
- 20.A-D ![]() )
)
Description.
Large fruticose thallus up to 14 mm long with protuberances up to 3.5 mm wide (Fig.
19.A ![]() ). VC
non-coaxial
(Fig. 19.B
). VC
non-coaxial
(Fig. 19.B ![]() ) with cells
12-20 µm long and 9-13 µm
wide. Typical cells in PF are rectangular, 11-16 µm in length and 8-11 µm in
diameter. Frequently, a distinct layer of flattened cells can be observed within
the peripheral region, or exactly below the sporangial compartments (Fig.
19.C
) with cells
12-20 µm long and 9-13 µm
wide. Typical cells in PF are rectangular, 11-16 µm in length and 8-11 µm in
diameter. Frequently, a distinct layer of flattened cells can be observed within
the peripheral region, or exactly below the sporangial compartments (Fig.
19.C ![]() ),
4-8 µm in length. Larger PF cells measure 18-25 µm in length. Epithallial
cells not observed. Secondary pit connections are abundant in PF and cell
fusions are also present (Fig. 19.B-C
),
4-8 µm in length. Larger PF cells measure 18-25 µm in length. Epithallial
cells not observed. Secondary pit connections are abundant in PF and cell
fusions are also present (Fig. 19.B-C ![]() ).
).
Sporangial compartments are cylindrical
to ovoid (Fig. 19.C-D ![]() ), up to 25 in a sorus and measure
102-158 µm in length
and 41-67 µm in diameter. Layer of elongated cells below the sporangial
compartments is absent. Paraphyses are 3-6 cells long (Fig.
19.C
), up to 25 in a sorus and measure
102-158 µm in length
and 41-67 µm in diameter. Layer of elongated cells below the sporangial
compartments is absent. Paraphyses are 3-6 cells long (Fig.
19.C ![]() ), the longest
measuring 22-30 µm in length and 5-8 µm in diameter.
), the longest
measuring 22-30 µm in length and 5-8 µm in diameter.
Two smaller sporangial compartments
measuring 77-95 µm in height and two gametangial conceptacles measuring 190-281
µm in diameter and 124-125 µm in height with pore canals not visible are
observed raised at the thallus surface (Fig. 19.D-F ![]() ).
).
Gametangial
conceptacles are also observed slightly raised in monoecious thallus that
encrusts Lithothamnion sp.1 (Figs. 11.A ![]() & 20.A
& 20.A ![]() ), in the same thin
section. VC hardly observable, with cells 14-20 µm in length and 8-12 µm in diameter. Cells in PF are
13-19
µm in length and 8-12 µm in diameter, similar to the ones in the thallus
bearing sporangial compartments. Cell fusions and secondary pit connections are
present (Fig. 20.B-D
), in the same thin
section. VC hardly observable, with cells 14-20 µm in length and 8-12 µm in diameter. Cells in PF are
13-19
µm in length and 8-12 µm in diameter, similar to the ones in the thallus
bearing sporangial compartments. Cell fusions and secondary pit connections are
present (Fig. 20.B-D ![]() ). The presumed female gametangial conceptacles
(Fig. 20.C
). The presumed female gametangial conceptacles
(Fig. 20.C ![]() )
measure 224-232
µm in diameter and 61-79 µm in height, with cylindrical pore canals 49-55 µm
wide and 73-96 µm long. The presumed male
conceptacles show a slightly convex floor (Fig. 20.D
)
measure 224-232
µm in diameter and 61-79 µm in height, with cylindrical pore canals 49-55 µm
wide and 73-96 µm long. The presumed male
conceptacles show a slightly convex floor (Fig. 20.D ![]() ) and measure
210-240 µm in
diameter and 39-44 µm in height.
) and measure
210-240 µm in
diameter and 39-44 µm in height.
Remarks. The identification of this species remains uncertain because of the presence of large sporangial compartments and the layer of flattened cells, which were not described from previous studies. Archaeolithothamnium sp. described from southern Poland (Pisera & Studencki, 1989) shows a similar growth form, biometric measurements of VC cells, PF cells and sporangial compartments. However, the number of cells in paraphyses cannot be observed in the illustrations.
Studied thin sections. LV 39, 60.
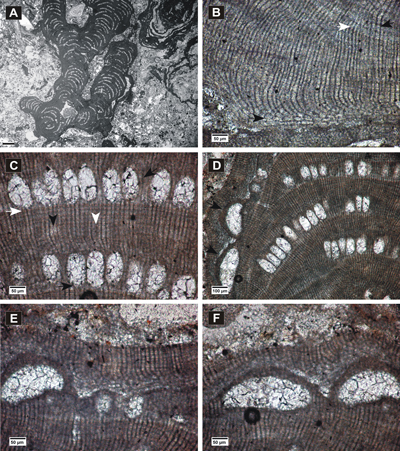
Click on thumbnail to enlarge the image.
Figure 19: Sporolithon sp. 1. A, fertile fruticose branch; B, non-coaxial ventral filaments, cell fusions in VC (black arrow) and in peripheral filaments (white arrow); C, layer of flattened cells below sporangial compartments (white arrow), cells in paraphyses (upper black arrow), stalk cell (lower black arrow), peripheral filaments showing cell fusion (black arrowhead) and secondary pit connections (white arrowhead); D, gametangial conceptacles protruding at the thallus surface (arrows); E, detail of gametangial conceptacle and two small sporangial compartments raised at the thallus surface; F, detail of gametangial conceptacles.

Click on thumbnail to enlarge the image.
Figure 20: Sporolithon sp. 1, gametangial thallus. A, monoecious gametangial thallus (double-headed arrow) encrusting Lithothamnion sp.1 and bearing uniporate conceptacles slightly raised at the thallus surface (white arrows); B-D, cell fusions (black arrows) and secondary pit connections (white arrows); B, peripheral filaments showing a PF cell arrangement similar to the one in thallus bearing sporangial compartments; C, presumed female gametangial conceptacle with cylindrical pore canal; D, presumed male conceptacles with a slightly convex floor.
The coralline algal assemblages from the Lopadea Veche section show a considerable diversification of species. Features that are used by phycologists to separate modern coralline species, have been identified in our material, and have proven to be effective for separating fossil species (Basso, 1994, 1995; Rasser & Piller, 1999; Iryu et al., 2012; Rebelo et al., 2014; Hrabovský et al., 2015).
One of the most important goals in the taxonomic studies of fossil corallines is to provide a full and accurate description of species, therefore, the identification of asexual, gametangial and carposporangial phases of a species is critical. The gametangial and carposporangial conceptacles occur only in uniporate chambers and, unlike modern specimens, the fossil corallines do not show preserved male and female gametes or carposporophytes. Therefore, the uniporate conceptacles are commonly misidentified as tetrasporangial conceptacles of thalli in asexual phase.
The male
conceptacles can be helpful in identifying coralline algal species if they are
found in association with the tetrasporangial thalli (Harvey et al.,
2005). Moreover, in case of fusion between the two types of thalli or when
published accounts of the gametangial thalli of species or genera are available
(Verheij, 1993; Hrabovský et al.,
2015), the
identification at species level is more feasible. In this study, the
identification of the gametangial phase of species was the most accessible when
taking notice of melobesioid thalli showing both multiporate and uniporate
conceptacles (Figs. 12.B ![]() ,
13.E
,
13.E ![]() & 14.B
& 14.B ![]() ). The male and the female gametangial
phase of mastophoroid species was obvious only for Spongites fruticulosus,
because of the published accounts of modern gametangial thalli (Basso
& Rodondi, 2006). The gametangial/carposporangial thalli of Sporolithon
sp. 1 (Figs. 19.D-F
). The male and the female gametangial
phase of mastophoroid species was obvious only for Spongites fruticulosus,
because of the published accounts of modern gametangial thalli (Basso
& Rodondi, 2006). The gametangial/carposporangial thalli of Sporolithon
sp. 1 (Figs. 19.D-F ![]() - 20.A-D
- 20.A-D ![]() ) and Sporolithon
praeerythraeum (Fig. 18.C-D
) and Sporolithon
praeerythraeum (Fig. 18.C-D ![]() ) were identified because of the presence of both
cell fusions and secondary pit connections and the resemblance to the thallus in
asexual phase. Moreover, similar presumed carposporangial conceptacles of S.
praeerythraeum are described from the paratype (Vannucci et al.,
2000) and gametangial conceptacles of Sporolithon sp. 1 are also found
protruding in the same thallus bearing sporangial compartments.
) were identified because of the presence of both
cell fusions and secondary pit connections and the resemblance to the thallus in
asexual phase. Moreover, similar presumed carposporangial conceptacles of S.
praeerythraeum are described from the paratype (Vannucci et al.,
2000) and gametangial conceptacles of Sporolithon sp. 1 are also found
protruding in the same thallus bearing sporangial compartments.
The red algal limestones from the Transylvanian Basin, Lopadea Veche section, show a great resemblance to the middle Miocene deposits of southern Poland. Nine of the identified species were also reported from the Korytnica Basin (Pisera & Studencki, 1989) and the distinguished facies types from Pińczów limestones (Studencki, 1988) were also recognized in the studied section. The coralline algal assemblages from the Badenian of Czech Republic were studied using similar taxonomic approaches (Hrabovský et al., 2015). Nevertheless, the resemblance of these two accounts is relatively low, with only four common species. Further detailed analysis of other sections in the Transylvanian Basin (Pietroasa, Podeni, Gârbova de Sus) can indicate different paleoenvironmental conditions that may be important in the paleogeographic distribution of coralline species.
Taking into account the stratigraphical and paleogeographical distribution of Lithothamnion crispatum (Coletti et al., 2016), Lithothamnion roveretoi (Vannucci et al., 2010; Hrabovský et al., 2015), Phymatolithon calcareum (Basso et al., 1997) and Sporolithon praeerythraeum (Vannucci et al., 2000), we conclude that during the middle Miocene, these species were distributed only in the Transylvanian Basin and other Central Paratethys basins. While S. fruticulosus, L. crispatum and P. calcareum are now common species in modern marine environments, S. praeeryhraeum apparently became extinct in the Mediterranean (Tethyan) domain by the end of the Paleogene (Vannucci et al., 2000; see also Vannucci et al., 2008, in respect to ? Lithophyllum perrandoi). However, this study indicates that the stratigraphical distribution of S. praeeryhraeum extended to the middle Miocene in the Transylvanian Basin.
Some diagnostic features commonly used for the identification and taxonomic description of modern coralline algae, were for the first time distinguished and used for describing middle Miocene specimens from the Transylvanian Basin, Romania.
This taxonomic study identified seventeen species belonging to Corallinales, Hapalidiales and Sporolithales. Gametangial/carposporangial thalli were identified and included in the description of six species (Spongites fruticulosus, Lithothamnion sp. 2, Lithothamnion sp. 3, ? Lithothamnion. sp. 4, Sporolithon praeerythraeum, Sporolithon sp. 1).
Female gametangial conceptacles of Spongites fruticulosus are for the first time identified and described in fossil material. We propose the assignment of Lithophyllum platticarpum Maslov to Spongites fruticulosus Kützing, as a gametangial thallus with male conceptacles because of their revealed similarities (growth form, conceptacles and biometric measurements) and the fusion with the thallus in asexual phase.
This paper is a result of a PhD research made possible by the financial support of the Sectoral Operational Programme for Human Resources Development 2007-2013, co-financed by the European Social Fund, under the project POSDRU/159/1.5/S/133391 - "Doctoral and postdoctoral excellence programs for training highly qualified human resources for research in the fields of Life Sciences, Environment and Earth". We are grateful to the reviewers J.C. Braga and D. Basso for suggestions that helped to improve this paper and to R.W. Scott and B. Ferré for the English revision and the French translation respectively.
Abrŕmof M.D., Magalhăes P.J. & Ram S.J. (2004).- Image processing with ImageJ.- Biophotonics International, Pittsfield, MA, vol. 11, no. 7, p. 36-42.
Adey W.H. & McKibbin D.L. (1970).- Studies on the maerl species Phymatolithon calcareum (Pallas) nov. comb. and Lithothamnium corallioides Crouan in the Ria de Vigo.- Botanica Marina, Berlin, vol. 13, p. 100-106.
Aguirre J. & Braga J.C. (1998).- Redescription of Lemoine's (1939) types of coralline algal species from Algeria.- Palaeontology, vol. 41, no. 3, p. 489-507.
Aguirre J., Braga J.C. & Bassi D. (2011).- Taxonomic assessment of coralline algal species (Rhodophyta: Corallinales and Sporolithales) described by Pfender, Lemoine, and Miranda from northern Spain type localities.- Annalen des Naturhistorischen Museums, Wien, (Serie A), Band 113, p. 267-289.
Aguirre J., Braga J.C. & Piller W.E. (1996).- Reassessment of Palaeothamnium Conti, 1946 (Corallinales, Rhodophyta).- Review of Palaeobotany and Palynology, vol. 94, p. 1-9.
Airoldi M. (1932).- Contributo allo studio delle Corallinacee del terziario italiano. I. Le Corallinacee dell'Oligocene ligure-piemontese.- Paleontographia Italica, Pisa, vol. 33, no. 3, p. 55-83.
Bassi D., Braga J.C., Zakrevskaya E. & Petrovna Radionova E. (2005).- Re-assessment of the type collection of corallinalean genera (Corallinales, Rhodophyta) described by V.P. Maslov.- Palaeontology, vol. 48, p. 929-945.
Bassi D., Braga J. C., Zakrevskaya E. & Petrovna Radionova E. (2007).- Redescription of the type collections of Maslov's species of Corallinales (Rhodophyta). II. Species included by Maslov in Archaeolithothamnium Rothpletz, 1891.- Revista Espańola de Paleontologia, Madrid, vol. 22, no. 2, p. 115-125.
Basso D. (1994).- Living calcareous algae by a paleontological approach: the non-geniculate Corallinaceae (Rhodophyta) of the soft bottoms of the Tyrrhenian Sea (Western Mediterranean). The genera Phymatolithon Foslie nom. cons. and Mesophyllum Lemoine.- Rivista Italiana di Paleontologia e Stratigrafia, Milano, vol. 100, no. 4, p. 575-596.
Basso D. (1995).- Living calcareous algae by a paleontological approach: the genus Lithothamnion Heydrich nom. cons. from the soft bottoms of the Tyrrhenian Sea (Mediterranean).- Rivista Italiana di Paleontologia e Stratigrafia, Milano, vol. 101, no. 3, p. 349-366.
Basso D., Fravega P., Piazza M. & Vannucci G. (1998).- Revision and re-documentation of M. Airoldi's species of Mesophyllum from the Tertiary Piedmont Basin (NW Italy).- Rivista Italiana di Paleontologia e Stratigrafia, Milano, vol. 104, no. 1, p. 85-94.
Basso D., Fravega P. & Vannucci G. (1996).- Fossil and living corallinaceans related to the Mediterranean endemic species Lithophyllum racemus (Lamarck) Foslie.- Facies, Erlangen, vol. 35, p. 275-292.
Basso D., Fravega P. & Vannucci G. (1997).- The taxonomy of Lithothamnium ramosissimum (Gümbel non Reuss) Conti and Lithothamnium operculatum (Conti) Conti (Rhodophyta, Corallinaceae).- Facies, Erlangen, vol. 37, p. 167-182.
Basso D. & Rodondi G. (2006).- A Mediterranean population of Spongites fruticulosus (Rhodophyta, Corallinales), the type species of Spongites, and the taxonomic status of S. stalactitica and S. racemosa.- Phycologia, Lawrence, vol. 45, no. 4, p. 403-416.
Basso D., Rodondi G. & Bressan G. (2011).- A re-description of Lithothamnion crispatum and the status of Lithothamnion superpositum (Rhodophyta, Corallinales).- Phycologia, Lawrence, vol. 50, no. 2, p. 144-155.
Basso D., Vrsaljko D. & Grgasović T. (2008).- The coralline flora of a Miocene maërl: the Croatian 'Litavac'.- Geologia Croatica, Zagreb, vol. 61, no. 2-3, p. 333-340.
Bizzozero G. (1885).- Flora Veneta Crittogamica. Parte II.- Seminario, Padova, 255 p.
Braga J.C. & Aguirre J. (2001).- Coralline algal assemblages in upper Neogene reef and temperate carbonates in Southern Spain.- Palæogeography, Palæoclimatology, Palæoecology, vol. 175, p. 27-41.
Braga J.C., Bassi D., Zakrevskaya E. & Petrovna Radionova E. (2005).- Reassessment of the type collections of Maslov's species of Corallinales (Rhodophyta). I. Species originally attributed to Lithophyllum and Melobesia.- Revista Espańola de Paleontología, Madrid, vol. 20, p. 207-224.
Braga J.C., Bosence D.W.J. & Steneck R. S. (1993).- New anatomical characters in fossil coralline algae and their taxonomic implications.- Palaeontology, vol. 36, p. 535-547.
Braga J.C., Vescogni A., Bosellini F.R. & Aguirre J. (2009).- Coralline algae (Corallinales, Rhodophyta) in western and central Mediterranean Messinian reefs.- Palæogeography, Palæoclimatology, Palæoecology, vol. 275, no. 1-4, p. 113-128.
Bucur I.I. & Filipescu S. (1994).- Middle Miocene red algae from the Transylvanian Basin (Romania).- Beiträge zur Paläontologie, Wien, vol. 19, p. 39-47.
Bucur I.I. & Filipescu S. (2011).- Middle Miocene red algae from Lopadea Veche (Western border of the Transylvanian Basin). In: Calcareous algae from Romanian Carpathians, Field Trip Guidebook, 10th International Symposium on Fossil Algae, Cluj Napoca, Romania.- Cluj University Press, p. 115-122.
Bucur I.I., Mészáros N. & Costea C. (1987).- Algues calcaires dans les dépôts de l'Éocčne supérieur de Prodănești et Cuciulat (NO du Bassin de Transylvanie). In: Petrescu I. (ed.), The Eocene from the Transylvanian Basin.- University of Cluj-Napoca, p. 31-36.
Bucur I.I., Saint-Martin J.-P., Filipescu S., Săsăran E. & Pleş G. (2011).- On the presence of green algae (Dasycladales, Bryopsidales) in the Middle Miocene deposits from Podeni (western border of the Transylvanian Basin, Romania).- Acta Palaeontologica Romaniae, Cluj Napoca, vol. 7, p. 69-75.
Ciulavu D. (1999).- Tertiary tectonics of the Transylvanian Basin.- PhD Thesis, Vrije Universiteit, Amsterdam, 152 p.
Coletti G., Basso D., Frixa A. & Corselli C. (2015).- Transported rhodoliths witness the lost carbonate factory: a case history from the Miocene Pietra da Cantoni limestone (NW Italy).- Rivista Italiana di Paleontologia e Stratigrafia, Milano, vol. 121, no. 3, p. 345-368.
Coletti G., Hrabovský J. & Basso D. (2016).- Lithothamnion crispatum: long-lasting species of non-geniculate coralline algae (Rhodophyta, Hapalidiales).- Carnets Geol., Madrid, vol. 16, no. 3, p. 27-41.
Conti S. (1946).- Le Corallinacee del calcare miocenico (Leithakalk) del bacino di Vienna.- Publicazioni del Instituto di Geologie dell'Universitŕ di Genova, (Serie A - Paleontologia), no. 1-2, p. 31-68.
Cronquist A. (1987).- The divisions and classes of plants.- The Botanical Review, vol. 26, p. 425-482.
Filipescu S. (1996).- Stratigraphy of the Neogene from the western border of the Transylvanian Basin.- Studia Universitatis Babeş - Bolyai, Cluj Napoca, (Geologia), vol. XLI, no. 2, p. 3-78.
Filipescu S. (2001).- Wielician foraminifera at the western border of the Transylvanian Basin.- Studia Universitatis Babeş - Bolyai, Cluj Napoca, (Geologia), vol. XLVI, no. 2, p. 115-123.
Filipescu S. & Gîrbacea R. (1997).- Stratigraphic remarks on the Middle Miocene deposits from Gârbova de Sus (Transylvanian Basin, Romania).- Studia Universitatis Babeş - Bolyai, Cluj Napoca, (Geologia), vol. XLI, p. 275-286.
Foslie M. (1898).- Systematical survey of the lithothamnia.- Det Kongelige Norske Videnskabers Selskabs Skrifter 1898, Trondheim, p. 1-7.
Foslie
M. (1906).- Algologiske notiser
II.- Det Kongelige Norske Videnskabers Selskabs Skrifter 1906,
Trondheim, no. 2, p. 1-34.
URL: http://www.ntnu.no/ojs/index.php/DKNVS_skrifter/article/view/1117/1035
Foslie M. (1909).-
Algologiske notiser. VI.- Det Kongelige
Norske Videnskabers Selskabs Skrifter 1909, Trondheim, no. 2, p. 1-63.
URL: http://www.ntnu.no/ojs/index.php/DKNVS_skrifter/article/view/1304/1213
Fravega P., Piazza M., Stockar R. & Vannucci G. (1994).- Oligocene coral and algal reef and related facies of Valzemola (Savona, NW Italy).- Rivista Italiana di Paleontologia e Stratigrafia, Milano, vol. 100, no. 3, p. 423-456.
Guiry
M.D. & Guiry G.M. (2016).-
Algaebase.- World-wide electronic
publication, National University of Ireland, Galway.
URL: http://www.algaebase.org/,
searched on 07 January 2016.
Harvey A.S., Broadwater S.T., Woelkerling W.J. & Mitrovski P.J. (2003).- Choreonema (Corallinales, Rhodophyta): 18S rDNA phylogeny and resurrection of the Hapalidiaceae for the subfamilies Choreonematoideae, Austrolithoideae, and Melobesioideae.- Journal of Phycology, vol. 39, p. 988-998.
Harvey A., Woelkerling W., Farr T., Neill K. & Nelson W. (2005).- Coralline algae of Central New Zealand.- NIWA Information Series, Wellington, no. 57, 145 p.
Hauck F. (1878).- Beiträge zur Kenntniss der Adriatischen Algen.- Österreichische Botanische Zeitschrift, Wien, Jahrgang XXVIII, p. 288-295.
Heydrich F. (1897).- Corallinaceae, insbesondere Melobesieae.- Berichte der deutsche botanischen Gesellschaft, Wien, Band XV, p. 34-70.
Hohenegger J., Ćorić S. & Wagreich M. (2014).- Timing of the Middle Miocene Badenian stage of the Central Paratethys.- Geologica Carpathica, Bratislava, vol. 65, no. 1, p. 55-66.
Hosu A. & Filipescu S. (1995).- Sequence stratigraphy on the Middle Miocene deposits from Gârbova de Sus Formation (Transylvanian Basin, Romania).- Studii și comunicări, Muzeul Județean Bistrița, vol. 1, p. 87-97.
Hrabovský J., Basso D. & Doláková N. (2015).- Diagnostic characters in fossil coralline algae (Corallinophycidae: Rhodophyta) from the Miocene of southern Moravia (Carpathian Foredeep, Czech Republic).- Journal of Systematic Palaeontology, vol. 14, no. 6, p. 1-27.
Iryu Y., Bassi D. & Woelkerling W.J. (2012).- Typification and reassessment of seventeen species of coralline red algae (Corallinales and Sporolithales, Rhodophyta) described by W. Ishijima during 1954-1978.- Journal of Systematic Palaeontology, vol. 10, no. 1, p. 171-209.
Johansen H.W. (1981).- Coralline algae. A first synthesis.- CRC Press, Boca Raton, Florida, 239 p.
Johnson J.H. (1964).- Paleocene calcareous red algae from northern Iraq.- Micropaleontology, vol. 10, no. 2, p. 207-216.
Kato A., Baba M. & Suda S. (2011).- Revision of the Mastophoroideae (Corallinales, Rhodophyta) and polyphyly in nongeniculate species widely distributed on Pacific coral reefs.- Journal of Phycology, vol. 47, p. 662-672.
Koch A. (1900).- Die Tertiärbildungen des Beckens der Siebenbürgische Landestheile. II Neogene Abtheilung.- Budapest, 370 p.
Kützing F.T. (1841).- Über die 'Polypiers calcifčres' des Lamouroux.- Zu der öffentlichen Prüfung sämmtlicher Classen der Realschule zu Nordhausen, p. 3-34.
Lamouroux J.V.F. (1812).- Sur la classification des Polypiers coralligčnes non entičrement pierreux.- Nouveau Bulletin des Sciences par la Société Philomatique de Paris, vol. 3, p. 181-188.
Le Gall L. & Saunders G.W. (2007).- A nuclear phylogeny of the Florideophyceae (Rhodophyta) inferred from combined EF2, small subunit and large subunit ribosomal DNA: establishing the new red algal subclass Corallinophycidae.- Molecular Phylogenetics and Evolution, vol. 43, p. 1118-1130.
Le Gall L., Payri C.E., Bittner C.E. & Saunders G.W (2009).- Multigene polygenetic analyses support recognition of the Sporolithales, ord. nov.- Molecular Phylogenetics and Evolution, vol. 54, p. 302-305.
Lemoine M. (1928).- Algues calcaires fossiles de l'Algérie.- Matériaux pour la Carte géologique de l'Algérie, Alger, (1čre série, Paléontologie), vol. 9, p. 1-128.
Leeuw A. de, Filipescu S., Maţenco L., Krijgsman W., Kuiper K. & Stoica M. (2013).- Paleomagnetic and chronostratigraphic constraints on the Middle to Late Miocene evolution of the Transylvanian Basin (Romania): Implications for Central Paratethys stratigraphy and emplacement of the Tisza - Dacia plate.- Global and Planetary Change, vol. 103, p. 82-98.
Lörenthey L. (1894a).- Bericht über die Resultate meiner geologischen Excursionen im Sommer 1891.- Orvos-Természettudományi, Értesitő, Cluj, vol. XV/I, p. 100-102.
Lörenthey L. (1894b).- Az erdélyi Múzeum-Egylet megbizásában 1891. Nyarán tett földtani kirándulásaimnak eredményeiröl.- Orvos-Természettudományi, Értesitő, Cluj, vol. XV/I, p. 55-68. [in Hungarian].
Lupu M., Borcoş M., Dimian M., Lupu D. & Dimitrescu R. (1967).- Turda sheet.- Geological Map of Romania scale 1:200.000, Geological Institute, Bucharest.
Maslov V.P. (1956).- Fossil calcareous algae of the USSR.- Trudy Instituta Geologicheskogo Nauk Akademii Nauk SSSR, vol. 160, 301 p. [in Russian].
Maslov V.P. (1962).- Fossil red algae of the USSR and their connections with facies.- Trudy Instituta Geologicheskogo Nauk Akademii SSSR, vol. 53, 222 p. [in Russian].
Mastrorilli V.I. (1973).- Flore fossili a corallinacee di alcune localita' venete tra I berici e l'altopiano di Asiago.- Atti della Societŕ Italiana di Scienze Naturali e del Museo Civico di storie natural di Milano, vol. 114, no. 3, p. 209-291.
Miranda F. (1935).- Algas coralináceas fósiles del Terciario de San Vicente de la Barquera (Santander).- Boletin de la Sociedad Espańola de Historia Natural, vol. 35, p. 279-287.
Nebelsick J.H. & Bassi D. (2000)-. Diversity, growth forms and taphonomy: key factors controlling the fabric of coralline algae dominated shelf carbonates.- Geological Society, Special Publications, London, vol. 178, p. 89-107.
Nebelsick J.H, Bassi D. & Drobne K. (2000).- Microfacies analysis and palaeoenvironmental interpretation of Lower Oligocene, shallow-water carbonates (Gornjia Grad Beds, Slovenia).- Facies, Erlangen, vol. 43, p. 157-176.
Nebelsick J.H., Bassi D. & Lempp J. (2013).- Tracking paleoenvironmental changes in coralline algal-dominated carbonates of the Lower Oligocene Calcareniti di Castelgomberto formation (Monti Berici, Italy). Facies, Erlangen, vol. 59, p. 133-148.
Nelson W.A., Suetherland J.E., Farr T.J., Hart D.R., Neill K.F., Kim J.J. & Yoon H.S. (2015).- Multi-gene phylogenetic analyses of New Zealand coralline algae: Corallinapetra novaezelandiae gen. et sp. nov. and recognition of the Hapalidiales ord. nov.- Journal of Phycology, vol. 51, no. 3, p. 454-468.
Orszag-Sperber F., Poignant A.F. & Poisson A. (1977).- Paleogeographic significance of rhodolites: some examples from the Miocene of France and Turkey. In: Flügel E. (ed.), Fossil Algae. Recent results and developments.- Springer Verlag, Berlin, p. 286-294.
Pallas P.S (1766).- Elenchus zoophytorum sistens generum adurnbrationes generaliores et specierum cognitarum succinctas descriptiones cum selectis auctorum synonymis.- Fransiscum Varrentrapp, Hagae, 451 p.
Pávay-Vajna F. (1910).- Die geologischen Verhaltnisse der Umgebung von Olah-Lapada.- Földtani Közlöny, Budapest, vol. XL, p. 420-434.
Penrose D. (1991).- Spongites fruticulosus (Corallinaceae, Rhodophyta), the type species of Spongites, in southern Australia.- Phycologia, Lawrence, vol. 30, no. 5, p. 438-448.
Piller W.E. (1994).- Nullipora ramosissima Reuss, 1847 - a rediscovery.- Beiträge zur Paläontologie, Wien, vol. 19, p. 181-189.
Pisera A. (1985).- Paleoecology and lithogenesis of the Middle Miocene (Badenian) algal-vermitid reefs from Roztocze Hills, south-eastern Poland.- Acta Geologica Polonica, Warszawa, vol. 35, no. 1-2, p. 89-155.
Pisera A. & Studencki W. (1989).- Middle Miocene rhodoliths from the Korytnica basin (Southern Poland): environmental significance and paleontology.- Acta Palaeontologica Polonica, Warszawa, vol. 34, p. 179-209.
Rasser M.W. (2000).- Coralline red algal limestones of the late Eocene Alpine Foreland Basin in Upper Austria: Component analysis, facies and paleoecology.- Facies, Erlangen, vol. 42, p. 59-92.
Rasser M.W. & Piller W.E. (1999).- Application of neontological taxonomic concepts to Late Eocene coralline algae (Rhodophyta) of the Austrian Molasse Zone.- Journal of Micropalaeontology, vol. 18, no. 1, p. 67-80.
Rebelo A.C., Rasser M., Riosmena-Rodríguez R., Neto A.I. & Ávila S. (2014).- Rhodolith forming coralline algae in the Upper Miocene of Santa Maria Island (Azores, NE Atlantic): a critical evaluation.- Phytotaxa, Auckland, vol. 190, no. 1, p. 370-382.
Reuss A.E. (1847).- Die fossilen Polyparien des Wiener Tertiärbeckens. Ein monographischer Versuch.- Naturwissenschaftlichen Abhandlungen, Magdeburg, vol. 2, 109 p.
Rosanoff S. (1866).- Recherches anatomiques sur les Mélobésiées (Hapalidium, Melobesia, Lithophyllum et Lithothamnion).- Mémoires de la Société Impériale des Sciences Naturelles de Cherbourg, vol. 12, p. 5-112.
Saint-Martin J.-P., Merle D., Cornée J.J., Filipescu S., Saint-Martin S. & Bucur I.I. (2007).- Les constructions coralliennes du Badénien (Miocčne moyen) de la bordure occidentale de la dépression de Transylvanie (Roumanie).- Comptes Rendus Palevol, Paris, vol. 6, p. 37-46.
Săndulescu M., Kräutner H., Borcoş M., Năstăseanu S., Patrulius D., Ștefanescu M., Ghenea C., Lupu M., Savu H., Bercia I. & Marinescu F. (1978).- Geological map of Romania 1: 1,000,000.- Institutul de Geologie şi Geofizică, Bucharest.
Setchell W.A. & Mason L.R. (1943).- Goniolithon and Neogoniolithon: two genera of crustaceous coralline algae.- Proceedings of the National Academy of Sciences, vol. 29, no. 3-4, p. 87-92.
Silva P.C. & Johansen H.W. (1986).- A reappraisal of the order Corallinales (Rhodophyceae).- British Phycological Journal, vol. 21, p. 245-254.
Studencki W. (1988).- Red algae from the Pinczow limestones (Middle Miocene, Swietokrzyskie Mountains, Poland).- Paleontologica Polonica, Warszawa, vol. 33, p. 3-57.
Şuraru N. (1992).- Biostratigraphische Erwängungen über das obere Badenian aus dem Gebiet von Lopadea Veche (Kreis Alba) - Pietroasa (Kreis Cluj).- Studia Universitatis Babeș-Bolyai, Cluj, (Seria Geologia-Geographia), vol. XXXVII, no. 2, p. 49-63.
Vannucci G., Piazza M., Fravega P. & Arnera V. (1994).- Le Rodoliti del Miocene inferiore del settore SW del Bacino Terziario del Piemonte (Spigno Monferrato - Alessandria).- Atti della Societŕ Toscana di Scienze Naturali Memorie, (serie A), vol. 100, p. 93-117.
Vannucci G., Piazza M., Fravega P. & Basso D. (2000).- Revision and re-documentation of M. Airoldi's species of Archaeolithothamnium from the Tertiary Piedmont basin (NW Italy).- Rivista Italiana di Paleontologia e Stratigrafia, Milano, vol. 106, no. 2, p. 191-202.
Vannucci G., Quaranta F. & Basso D. (2008).- Revision and re-documentation of M. Airoldi's species of Lithophyllum from the Tertiary Piedmont Basin.- Rivista Italiana di Paleontologia e Stratigrafia, Milano, vol. 114, no. 3, p. 515-528.
Vannucci G., Quaranta F. & Basso D. (2010).- Revision and re-documentation of M. Airoldi's species of Lithothamnion from the Tertiary Piedmont basin (NW Italy).- Rivista Italiana di Paleontologia e Stratigrafia, Milano, vol. 116, no. 2, p. 223-235.
Verheij E. (1993).- The genus Sporolithon (Sporolithaceae fam. nov., Corallinales, Rhodophyta) from the Spermonde Archipelago, Indonesia.- Phycologia, Lawrence, vol. 32, no. 3, p. 184-196.
Wettstein R.R. (1901).- Handbuch der systematischen Botanik, vol. 1.- Deuticke, Leipzig, 201 p.
Woelkerling W.J. (1988).- The coralline red algae: an analysis of the genera and subfamilies of nongeniculate Corallinaceae.- Oxford University Press, 268 p.
Woelkerling W.J. & Irvine L.M. (1986).- The typification and status of Phymatolithon (Corallinaceae, Rhodophyta).- British Phycological Journal, vol. 21, p. 55-88.
Woelkerling W.J., Irvine L.M. & Harvey A.S. (1993).- Growth-forms in non-geniculate coralline red algae (Corallinales, Rhodophyta).- Australian Systematic Botany, Clayton South, vol. 6, p. 277-293.
Zágoršek K., Filipescu S. & Holcová K. (2010).- New Middle Miocene Bryozoa from Gârbova de Sus (Romania) and their relationship to the sedimentary environment.- Geologica Carpathica, Bratislava, vol. 61, no. 6, p. 495-512.