Contents
[Introduction] [Material and methods]
[Results]
[Discussion] [Bibliographic references] [Table] and ...
[Figures]
* Auteur correspondant - Corresponding author
Department of Geology, University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
The Australian Museum, 6 College Street Sydney 2010 NSW (Australia)
Manuscript online since October 10, 2011
Although the walls of most serpulid tubes are homogeneous, tubes of certain species may contain up to four ultrastructurally distinct layers. Some of these layers are made of densely packed large crystals and others are composed of sparsely packed fine crystals. In almost all (16 of 17) examined species having layered tubes, the dense layer is located in the outer wall part and the layer(s) composed of fine and relatively sparsely packed crystals are positioned in the inner wall part. Two species have transparent tube walls made entirely of densely packed crystals. Fossil serpulid tubes with dense outer layers (DOL) are known from the Late Cretaceous (Pentaditrupa subtorquata) and the Eocene (Pyrgopolon cf. mellevillei and Rotularia spirulaea). DOL gives a characteristic smooth shiny appearance to the tube surface and presumably evolved as an adaptation against drilling predation by gastropods and to delay shell dissolution in the waters of the deep-sea under-saturated with calcium carbonate.
Serpulidae; biomineralization; tube ultrastructure; evolution.
O. & E.K. (2011).- Evolution of a dense outer protective tube layer in serpulids (Polychaeta, Annelida).- Carnets de Géologie - Notebooks on Geology, Brest, Letter 2011/05 (CG2011_L05), p. 137-147.
Évolution de la couche externe, dense et protectrice, du tube de Serpulidés (Polychètes, Annélides).- Alors que les parois de la plupart des tubes de Serpulidés sont homogènes, les tubes de quelques espèces peuvent présenter jusqu'à quatre couches à ultrastructures variées. Quelques-unes de ces couches sont constituées de gros cristaux compacts et d'autres de fins cristaux épars. Chez presque toutes les espèces étudiées ayant un tube stratifié (16 sur 17), la couche compacte se situe dans la partie externe de la paroi minéralisée du tube ; quant à celle(s) constituée(s) de cristaux fins et présentant un agencement relativement lâche, elle(s) se trouve(nt) dans la partie interne de la paroi. Deux espèces présentent des parois transparentes constituées de cristaux compacts. Les tubes de Serpulidés fossiles à couches externes compactes ("dense outer layers", DOL) sont connus dès le Crétacé supérieur avec Pentaditrupa subtorquata, et à l'Éocène avec, par exemple, Pyrgopolon cf. mellevillei et Rotularia spirulaea. Cette ultrastructure, DOL, donne un aspect lisse et brillant, caractéristique, à la surface du tube et pourrait résulter d'une adaptation pour contrer la prédation par les Gastéropodes perforants et pour retarder la dissolution de la paroi minéralisée dans des eaux sous-saturées en carbonate de calcium.
Serpulidae ; biominéralisation ; ultrastructure du tube ; évolution.
Serpulids are polychaetes that build elaborate calcareous tubes. This large and highly successful group has a cosmopolitan geographic and wide bathymetric distribution, from the intertidal to the abyssal zones (ten & , 2009; et alii, 2011, & , 2011) and its representatives are even found in chemosynthetic communities of hydrocarbon seeps and hydrothermal vents ( et alii, 2010). Some taxa (of the genus Ficopomatus) tolerate a wide range of salinities (ten & , 2009) and one species, Marifugia cavatica, inhabits the fresh waters of subterranean caves ( et alii, 2009).
Serpulid tubes are composed of either calcite (most stable common natural polymorph of CaCO3), aragonite (another common polymorph of CaCO3), or a combination of both (, 1954; & , 1973; & , 1989; et alii, 1991; et alii, 2008a, b, c). Because of their calcareous tubes, serpulids may have played an important role in the ocean carbonate sink (both ongoing and past) as their tubes contribute to the sedimentary rock formation ( & , 1975; ten & Van den , 1993; et alii, 2000; & , 2009). The group has the best fossil record among all annelids. The earliest undisputable records of serpulids belong to the Middle Triassic, while earlier Palaeozoic records of the serpulids belong to various groups of problematic tubeworms ( & , 1977; , 1994; & , 2005, 2009; , 2006, 2010; & , 2007; & , 2008).
The tubes of serpulids have a relatively simple external macromorphology. Internally, the mineral tubes are lined with a thin organic layer (, 1993; , 2011), but a thick external organic (conchiolin) layer, such as found in the periostracum of molluscs and brachiopods is absent. On the inside, the tube surface is usually smooth, although some species form internal transverse tabulae (, 1958; ten , 1973; ten & , 1990), or rarely, longitudinal keels (Spiraserpula: & ten , 1994). Growth lamellae, the units of tube wall produced during a single secretion episode, are commonly chevron shaped in longitudinal section (, 1994; & , 2008) or, more rarely, are straight bands (, 1996). At the point of contact between the lateral tube wall and the substrate, the tube may produce cavities known as alveolar structures (e.g., , 1958).
Externally, serpulid tubes may bear additional sculpturing in the form of smooth or spiny longitudinal keels and transverse ridges or peristomes (, 1964; ten , 1973, 1975; & ten , 2002; et alii, 1995; ten & , 2009). The surface of most tubes shows faint growth striations, however, in a number of species it is smooth and appears to be covered with a transparent layer that gives the tube porcelain appearance ( & , 2008; ten & , 2009).
In contrast to relatively simple morphology, Recent and fossil serpulid tubes may show surprising ultrastructural complexity (, 2007; & , 2008; et alii, 2008a, b, c, d): at least thirteen distinct tube ultrastructures involving unoriented, uniformly oriented, or complex oriented crystals have been described ( et alii, 2008b, d). Although most serpulid tubes are single-layered, there can be up to four ultrastructurally distinct layers ( et alii, 2008b).
There are two controversial models of biomineralization in serpulids. According to the traditional model ( & , 1989), the calcareous granules excreted as slurry from the glands underneath the collar solidify slowly, allowing the folded collar to mould the calcite-saturated mucus into the end tube part. However, because this model cannot explain the observed ultrastructural complexity of the serpulid tubes, recent studies of tube ultrastructure ( et alii, 2008b, 2009) and biochemistry ( et alii, 2010) resulted in new models that explain biomineralization as a organic matrix-mediated process.
The aim of the study was to examine whether there is a pattern in the position of various tube layers. We also discuss functions of the outer tube layer and potential trends in evolution of dense layers in serpulid tubes.
This study is based on an extensive database containing scanning electron micrographs (SEM) of tube ultrastructures of 45 globally distributed Recent and 20 fossil serpulid species ranging in age from the Lower Jurassic through the Pliocene of Europe (, 2009; , 2005, 2007; et alii, 2008b, c, d). The database was examined to determine the structural differences between the outer and inner parts of the serpulid tube wall (Table 1). Recent material was collected intertidally or by diving, trawling, or dredging; it was fixed in 4% (buffered) formalin and was later transferred to 70% ethanol for museum storage. The material was deposited in Zoological Museum, University of Amsterdam, the Netherlands (ZMA), now in the process of being merged with the Netherlands Centre for Biodiversity, Naturalis, Leyden. Serpulid species were selected for the study in order to cover most of the accepted genera.
All samples with a mineralized ultrastructure (= tube fragments) were embedded in epoxy resin, polished in longitudinal and transversal directions, and then etched with 1% acetic acid for five to ten minutes prior to scanning electron microscope (SEM) examination. We used 5-15-mm-long longitudinal sections, and between one and three cross sections of each serpulid species. Some samples were repolished and treated with a 1:1 mixture of 25% glutaraldehyde and 1% acetic acid, to which alcian blue was added ( solution) before performing the SEM study ( et alii, 2005). As a result of this treatment, the organic-rich parts of the tubes have a more intensive blue color. Some samples were repolished and bleached with NaClO for removing organic matrixes before the SEM study. Scanning electron microscopy was done with a Hitachi S-4300, equipped with an Inca EDX system (energy-dispersive X-ray spectroscope), at the Swedish Museum of Natural History, Stockholm, and the etched samples and organic lining were viewed with a Zeiss 940D microscope, equipped with SAMx SDD EDX (energy-dispersive X-ray spectroscope), at the Department of Geology, University of Tartu. The beam was operated at 5-10 kV and 1 nA.
Evolution of serpulid shell layers was also analyzed using a published cladogram of serpulid relationships ( & , 2010). This phylogenetic tree was selected for the present study because it covers the best range of genera of this paper.
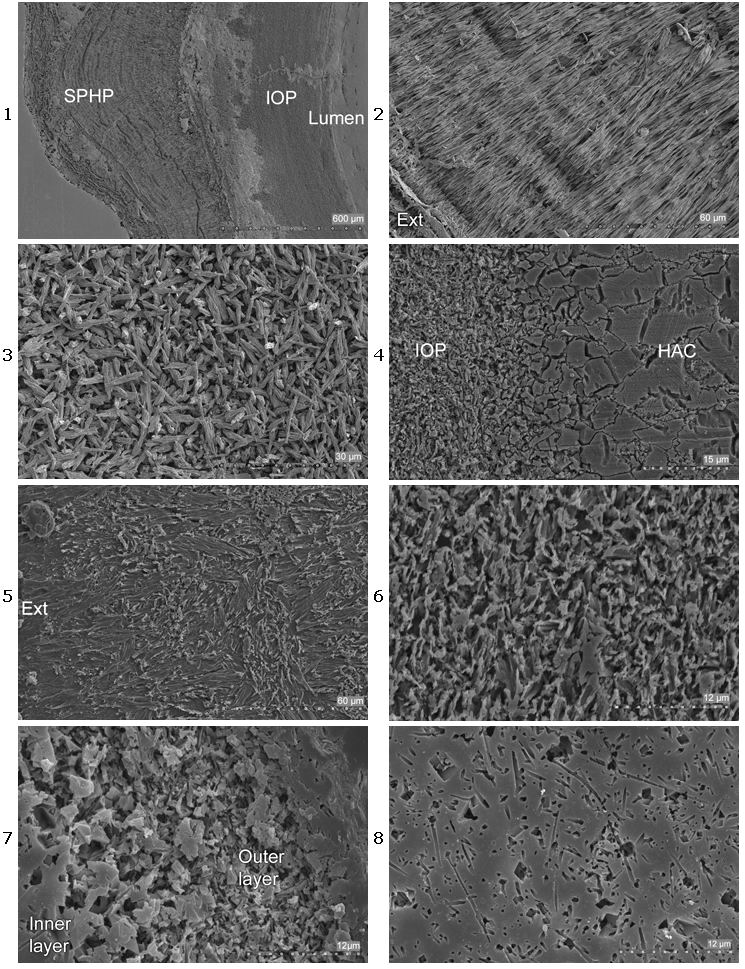
Out of 50 examined Recent and fossil species, tubes of 31 contained a single layer made of mostly isotropic (homogeneous or irregularly oriented, but see below) ultrastructures, while 17 had more than one layer (Table 1). The tube structures of those species that had two or more tube layers involved one layer made of large densely packed crystals and layer(s) composed of relatively sparsely packed fine crystals. In 16 of those 17 species (Table 1) with multilayered tubes, the dense layer was located in the outer tube wall (Figs.
1-2 & 4-5 ![]() ), contrasting with the inner layers made of finer and more sparsely packed crystals (Figs.
3 & 6
), contrasting with the inner layers made of finer and more sparsely packed crystals (Figs.
3 & 6 ![]() ). The situation is opposite only in the Recent Marifugia cavatica (Figs. 7 & 8
). The situation is opposite only in the Recent Marifugia cavatica (Figs. 7 & 8 ![]() ), which had less dense layer positioned in the outer tube wall. Tubes of two species (of 31 single-layered species), Placostegus tridentatus and Vitreotubus digeronimoi, were unusual in having the entire tube walls made of large densely packed crystals of relatively same size and arrangement.
), which had less dense layer positioned in the outer tube wall. Tubes of two species (of 31 single-layered species), Placostegus tridentatus and Vitreotubus digeronimoi, were unusual in having the entire tube walls made of large densely packed crystals of relatively same size and arrangement.
The dense outer layers (DOL) made of large crystals were arranged both into oriented (spherulitic prismatic, simple prismatic, regularly ridged prismatic) and unoriented (homogeneous angular crystal, and irregularly oriented platy) structures according to et alii (2008b,
d) (Figs. 1-2 & 4-5 ![]() ; Table 1). DOLs appeared transparent in tubes of Recent species. This transparency is presumably caused by large size of crystals, especially when crystals are densely packed with little organic material between them. However, uniform crystal orientation probably also supports optical transparency. Transparent outer layers varied in thickness, ranging from very thin as found in Crucigera websteri (1/15 of tube wall thickness) to very thick as in Ditrupa (> ½ of tube wall thickness). Moreover, tubes of two species, Placostegus tridentatus, and Vitreotubus digeronimoi that have completely transparent tubes were made entirely of densely packed large crystals arranged into simple prismatic structure.
; Table 1). DOLs appeared transparent in tubes of Recent species. This transparency is presumably caused by large size of crystals, especially when crystals are densely packed with little organic material between them. However, uniform crystal orientation probably also supports optical transparency. Transparent outer layers varied in thickness, ranging from very thin as found in Crucigera websteri (1/15 of tube wall thickness) to very thick as in Ditrupa (> ½ of tube wall thickness). Moreover, tubes of two species, Placostegus tridentatus, and Vitreotubus digeronimoi that have completely transparent tubes were made entirely of densely packed large crystals arranged into simple prismatic structure.
Serpulids with dense tube outer layer (oriented: spherulitic prismatic) are known from the Cretaceous in Pentaditrupa subtorquata (Table 1) and from the Eocene in Pyrgopolon cf. mellevillei (Figs. 1-3 ![]() ) and in Rotularia spirulae.
) and in Rotularia spirulae.
Sparsely packed fine crystals of inner tube layers were organized into isotropic (irregularly oriented prismatic, spherulitic irregularly oriented prismatic and fine homogeneous angular crystal) structures according to et alii (2008b) (Fig. 3 & 6 ![]() ).
).
Our study found that different types of serpulid tube ultrastructures are not randomly located in the serpulid tubes made of two or more layers. In all studied species, except for the freshwater cave-dwelling species Marifugia cavatica, more dense tube layers are located in the outer part of the tube wall (Table 1). That Marifugia shows a reverse organization of layers could be an adaptation to life in oversaturated in calcium carbonate freshwaters of limestone caves. We hypothesize that the location of the tube layers in other studied serpulids (all marine) may indicate an evolutionary adaptation of serpulid polychaetes to counteract predation and carbonate dissolution.
The calcareous exoskeletons of many marine invertebrates, such as molluscs ( & , 1969), brachiopods ( & , 1978), and bryozoans (, 1968) are externally covered by an organic layer. This layer isolates mineral parts of the calcareous structures from sea water and could protect against carbonate dissolution and predation ( & , 1969; , 1997; , 1998). Serpulid tubes lack an external organic cover and are in direct contact with seawater that can dissolve structures made of calcium carbonate. The rate of such dissolution in coastal environments, although normally insignificant, is inversely related to temperature (, 1997). Pressure alone does not affect the calcite solubility as much as temperature does, but when pressure is significant, its effect alone can increase calcite solubility about two fold ( & , 1995). In the deep sea, calcium carbonate accumulation is costly because of low temperatures and tremendous pressures. Dissolution of biogenic carbonate begins at several hundred meters and increases only gradually until the lysocline, a zone of rapid dissolution. Below the lysocline, carbonate compensation depth (CCD, 4200-5000 m) is reached at which the rate of carbonate accumulation equals the rate of carbonate dissolution, thus, calcareous skeletons of marine animals accumulate at the same rate at which they dissolve (, 1997; & , 2004). Serpulid inhabit even the deepest parts of the Ocean ( et alii, 2011), where carbonate dissolution is intense, and should presumably need special adaptations, both physiological and structural, against carbonate dissolution of their tubes.
Although serpulids are protected by the tubes and the opercula that block the tube entrance, they are attacked by drilling and crushing predators, such as crabs ( et alii, 2004), echinoids, asteroids, fish (, 1979), and especially naticid gastropods (ten , 1994; & , 1998; & , 2009).
The outer organic layers (conchiolin or periostracum) of bivalves are widely believed to be an adaptation that inhibits drilling by predatory naticid gastropods (see , 1998 and references therein). Such predation may be similarly discouraged by harder external mineral tube layers of serpulids. The most plesiomorphic serpulid tubes presumably had aragonitic irregularly oriented prismatic (IOP) ultrastructure ( et alii, 2008b, c) composed of fine unoriented prismatic crystals in an organic matrix, similar to that known for "Serpula" etalensis from the Early Jurassic ( et alii, 2008c). The earliest signs of drilling predation on serpulids (Placostegus) presumably by gastropods are reported from the Late Cretaceous ( & , 2007; see also ten , 1994). The earliest serpulids with two-layered tubes, containing a dense outer layer and less dense inner layers occur in the Cretaceous (Pentaditrupa subtorquata: , 2005). It appears that in addition to dissolution risk by sea water, serpulids have had to tolerate predation pressure throughout most of their evolutionary history. The appearance of species with DOLs in the Mesozoic could reflect an adaptation against predation because this era, particularly its Jurassic and Cretaceous periods, was a time of increased predation intensities, especially in the shallow water habitats (, 1996). This event, termed by (1977) as Mesozoic Marine Revolution, may well be associated with the appearance of presumable defensive tube structures in serpulids.
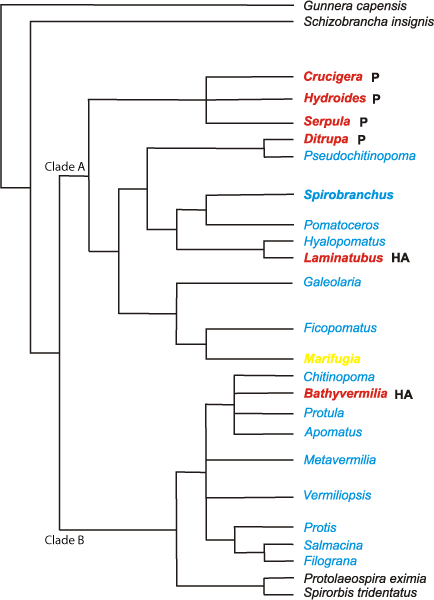
Our study showed that the distribution of Recent serpulid tubes with and without DOLs does not seem to show any ecological pattern, as animals with DOLs are found in all habitats (Table 1). The explanation of the observed pattern appears to be found in the evolutionary history of the group. Phylogenetic studies by et alii (2006,
2009) and & (2010) consistently indicate that serpulids are split into two major clades A and B (Fig. 9 ![]() ). Serpulid taxa in Clade A are characterized by the most advanced and complex oriented tube ultrastructures such as lamello-fibrillar, ordered fibrillar, and spherulitic lamello-fibrillar structures ( et alii,
2008b). Clade B is characterized by isotropic (non-oriented) structures, mostly by irregularly oriented prismatic and spherulitic irregularly oriented prismatic structures. Formation of these structures requires less biological control over the mineralization process than formation of complex oriented ultrastructures in the tubes of clade A ( et alii,
2008b). Thus, taxa in Clade A have a more advanced biomineralization system than those in the Clade B, the latter appear to be more close to the primitive plesiomorphic condition of serpulid biomineralization system. Although DOLs in the serpulid tubes occur in both major clades of serpulids (Fig. 9
). Serpulid taxa in Clade A are characterized by the most advanced and complex oriented tube ultrastructures such as lamello-fibrillar, ordered fibrillar, and spherulitic lamello-fibrillar structures ( et alii,
2008b). Clade B is characterized by isotropic (non-oriented) structures, mostly by irregularly oriented prismatic and spherulitic irregularly oriented prismatic structures. Formation of these structures requires less biological control over the mineralization process than formation of complex oriented ultrastructures in the tubes of clade A ( et alii,
2008b). Thus, taxa in Clade A have a more advanced biomineralization system than those in the Clade B, the latter appear to be more close to the primitive plesiomorphic condition of serpulid biomineralization system. Although DOLs in the serpulid tubes occur in both major clades of serpulids (Fig. 9 ![]() ), the majority of them (16 out of 17) are found in the apomorphic clade A that contains the most speciose and ecologically successful shallow-water serpulid genera such as Hydroides and Serpula. DOLs are absent in several unusual species of that clade, such as brackish-water species of the genus Ficopomatus, fresh-water Marifugia cavatica,
and coral-embedded Floriprotis sabiuraensis and Hydroides spongicola. The external tube walls of three other species from clade A lacking DOLs, Serpula vermicularis, S. israelitica and Spirobranchus triqueter, are composed of complex oriented structures that also have enhanced mechanical properties as compared to the isotropic structures of clade B ( et alii,
2008b). Placostegus tridentatus and Vitreotubus digeronimoi, the only taxa with tubes exclusively made of dense structures, also belong to Clade A.
), the majority of them (16 out of 17) are found in the apomorphic clade A that contains the most speciose and ecologically successful shallow-water serpulid genera such as Hydroides and Serpula. DOLs are absent in several unusual species of that clade, such as brackish-water species of the genus Ficopomatus, fresh-water Marifugia cavatica,
and coral-embedded Floriprotis sabiuraensis and Hydroides spongicola. The external tube walls of three other species from clade A lacking DOLs, Serpula vermicularis, S. israelitica and Spirobranchus triqueter, are composed of complex oriented structures that also have enhanced mechanical properties as compared to the isotropic structures of clade B ( et alii,
2008b). Placostegus tridentatus and Vitreotubus digeronimoi, the only taxa with tubes exclusively made of dense structures, also belong to Clade A.
The only taxon in plesiomorphic Clade B having two-layered tubes with DOL is the abyssal Bathyvermilia langerhansi. The dense outer layer in this species is very similar in structure to that found in the tube of Laminatubus alvini, a deep-sea species from clade A. Thus, it is possible that similar (homogeneous angular crystal structure) dense outer layers in tubes of Bathyvermilia and Laminatubus have independently evolved as adaptations to counteract tube dissolution in abyssal depths. In abyssal depth sea water is under saturated of calcium carbonate and skeletons made of CaCO3 are being dissolved much faster than in shallower depths due to cold temperature and high pressure.
Thus, we suggest that DOLs evolved as a response to increased predation pressure in relatively shallow-water serpulids of the apomorphic clade A, and as a separate adaptation to calcium carbonate dissolution in deep-sea representatives of the plesiomorphic clade B. A detailed phylogeny of the group and additional mineralogical studies involving tubes of abyssal serpulid species are needed to test the hypothesis.
We are grateful to journal reviewers Harry A. ten and Harry for their constructive reviews. O.V. is indebted to the grant (Paleontological Society) and the target-financed project (from the Estonian Ministry of Education and Science) SF0180051s08 (Ordovician and Silurian climate changes, as documented from the biotic changes and depositional environments in the Baltoscandian Palaeobasin) for financial support.
S., C.N. & C. (1995).- Scanning electron microscope observations on the tube of the reef-forming serpulid Ficopomatus enigmaticus () (Annelida, Polychaeta).- Bolletino Zoologico, Abingdon, vol. 62, p. 363-367.
W.C. (1968).- The body wall of cheilostome bryozoa. I. The ectocyst of Watersipora nigra ( and ).- Journal of Morphology, Malden, vol. 125, p. 497-507.
J.R. & H.A. ten (2002).- Revision of Hydroides , 1768 (Polychaeta: Serpulidae) from the western Atlantic region.- Beaufortia, Amsterdam, vol. 52, p. 103-178.
B.D. & J.D. (1973).- Generic and environmental control of carbonate mineralogy in serpulid (polychaete) tubes.- The Journal of Geology, Chicago, vol. 81, p. 363-373.
D.W.J. (1979).- The factors leading to aggregation and reef formation in Serpula vermicularis . In: G. & B.R. (eds.), Biology and Systematics of Colonial Organisms.- Systematics Association Special Volume, vol. 11, Academic Press, London and New York, p. 299-318.
T.P. & R. (1977).- Attached vermiform gastropods in Carboniferous marginal marine stromatolites and biostromes.- Lethaia, Oslo, vol. 10, p. 17-28.
E.M. (1997).- The molluscan periostracum: an important constraint in bivalve evolution.- Palaeontology, London, vol. 40, p. 71-97.
R.H. (1958).- Tube formation by Pomatoceros triqueter (Polychaeta).- Journal of the Marine Biological Association of the United Kingdom, Plymouth, vol. 37, p. 315-322.
H.A. ten (1973).- Serpulinae (Polychaeta) from the Caribbean: II - the genus Sclerostyla.- Studies on the Fauna of Curaçao and other Caribbean Islands, Utrecht, vol. 139, p. 1-21.
H.A. ten (1975).- Serpulinae (Polychaeta) from the Caribbean: III - the genus Pseudovermilia.- Studies on the Fauna of Curaçao and other Caribbean Islands, Utrecht, vol. 156, p. 46-101.
H.A. ten (1994).- The dualistic relation between molluscs and serpulid tube-worms. In: M. et alii (eds.), De horen en zijn echo.- Stichting Libri Antilliani, Zoölogisch Museum Amsterdam, p. 65-70.
H.A. ten & P. van den (1993).- A review of recent and fossil serpulid 'reefs': actuopalaeontology and the 'Upper Malm' serpulid limestone in NW Germany.- Geologie en Mijnbouw, Amsterdam, vol. 72, p. 23-67.
H.A. ten & E.K. (2009).- Taxonomy of Serpulidae (Annelida, Polychaeta): the state of affairs.- Zootaxa, Auckland, vol. 2036, 126 p. http://www.mapress.com/zootaxa/2009/f/zt02036p126.pdf
H.A. ten & R.S. (1990).- A re-description of Ditrupa gracillima , 1878 (Polychaeta, Serpulidae) from the Indo-Pacific, with a discussion of the genus.- Records of the Australian Museum, Sydney, vol. 42, p. 101-118.
A.P. & A.V. (2008).- On the Microstructure of tubes of modern spirorbids (Annelida, Polychaeta).- Doklady Biologicheskih Nauk, Moscow, vol. 418, p. 20-22.
M. & T. (2007).- A new serpulid, Placostegus velimensis sp. nov., from the Lower Turonian of the Bohemian Cretaceous Basin.- Acta Geologica Polonica, Warszawa, vol. 57, p. 89-96.
G. (1998).- Evidence from the fossil record of an antipredatory exaptation: conchiolin layers in corbilid bivalves.- Evolution, Malden, vol. 52, p. 68-79.
K.B. & D.K. (1995).- Introduction to geochemistry (3rd ed.).- McGraw-Hill Inc., New York, 647 p.
E.K., J. & E. (2011).- New records of Serpulidae (Annelida, Polychaeta) collected by R/V "Vityaz" from bathyal and abyssal depths of the Pacific Ocean.- Zootaxa, Auckland, vol. 2871, p. 43-60.
E.K., H.A. ten, B., V., P. & G.W. (2009).- Evolution of the unique freshwater cave-dwelling tube worm Marifugia cavatica (Annelida: Serpulidae).- Systematics and Biodiversity. London, vol. 7, p. 389-401.
E. K., T.A. & G.W. (2006).- Phylogenetic relationships within Serpulidae (Annelida: Polychaeta) inferred from molecular and morphological data. - Zoologica Scripta, Stockholm, vol. 35, p. 421-439.
E.K. & E. (2010).- Serpulidae (Annelida, Polychaeta) from Patton-Murray Seamounts, Gulf of Alaska, North Pacific Ocean.- Zootaxa, Auckland, vol. 2665, p. 51-68.
E.K. & E. (2011).- New records of the deep-sea serpulid polychaete Nogrobs grimaldii.- Marine Biodiversity Records. (In press)
E.K., E., M. & Y. (2010).- New records of Serpulidae (Annelida, Polychaeta) from hydrothermal vents of North Fiji, Pacific Ocean.- Zootaxa, Auckland, vol. 2389, p. 57-68.
D. (1997).- Aqueous environmental geochemistry.- Prentice-Hall, Inc., Upper Sanddle River, NJ, 600 p.
H.A. (1954).- Environmental relations of modification compositions of certain carbonate secreting marine invertebrates.- Proceedings of the National Academy of Sciences of the USA, Washington, vol. 40, p. 39-48.
P. & L. (1975).- Sedimenti calcareoargillosi e biolititi a serpulidi nel Mar Piccolo di Taranto.- Bolletino della Società geologica italiana, Roma, vol. 94, p. 2019-2046.
E., A., C., F., J. & M. (2000).- Population dynamics, secondary production and calcification in a Mediterranean population of Ditrupa arietina (Annelida: Polychaeta).- Marine Ecology Progress Series, Oldendorf, vol. 199, p. 171-184.
B. & E.M. (2009).- Drilling predation upon Ditrupa arietina (Polychaeta: Serpulidae) from the Mid-Atlantic Açores, Portugal.- Açoreana, Suplemento, Açores, vol. 6, p. 157-165.
E. (1993).- On the internal structure of calcified tube walls in Serpulidae and Spirorbidae (Annelida, Polychaeta).- Marine Fouling, Iwate, vol. 10, p. 17-20.
T. (1996).- Is predation intensity reduced with increasing depth? Evidence from the West Atlantic stalked crinoid Endoxocrinus parrae () and implications for the Mesozoic Marine Revolution.- Paleobiology, Tulsa, vol. 22, p. 339-351.
T.G. & H.A. ten (1994).- On recent species of Spiraserpula , 1961, a serpulid polychaete genus hitherto known only from Cretaceous and Tertiary fossils.- Bulletin Natural History Museum London (Zoology), London, vol. 60, p. 39-104.
E.S., D.J. & M.T. (2004).- Underwater television observations of Serpula vermicularis () reefs and associated mobile fauna in Loch Creran, Scotland.- Estuarine, Coastal and Shelf Science, London, vol. 61, p. 425-435.
H. (1964).- "Wurm-" und Serpuliden-Röhren in Geschieben unter besonderer Berücksichtigung von "Riffbildungen".- Lauenburgische Heimat, Neue Folge, Ratzeburg, vol. 45, p. 57-62.
R. (1996).- Micromorphology, microstructure and functional morphology of the Josephella marenzelleri (Polychaeta Serpulidae) tube. In: A. (ed.), Autoecology of selected organisms: Achievements and problems.- Bolletino della Società Paleontologica Italiana, Special Volume, Modena, vol. 3, p. 205-211.
R. (2009).- New species of Hyalopomatus , 1878 (Annelida, Polychaeta, Serpulidae) from Recent Mediterranean deep-water coral mounds and comments on some congeners.- Zoosystema, Paris, vol. 31, p. 147-161.
K. & K.M. (1989).- Biomineralization: cell biology and mineral deposition.- Academic Press, San Diego, 337 p.
B.R., E., J. & M. (2005).- 's solution: an ideal agent for resolving microgrowth structures of biogenic carbonates.- Palæogeography, Palæoclimatology, Palæoecology, Amsterdam, vol. 228, p. 149-166.
K.S. & B. (1998).- The ecology of Engina armillata (Gastropoda: Buccinidae) in the Cape d'Aguilar Marine Reserve, Hong Kong, with particular reference to its preferred prey (Polychaeta: Serpulidae).- Journal of Zoology, London, vol. 244, p. 391-403.
A.E., N., R.M.A., C.J. & G.C. (2010).- Insights into the composition, morphology, and formation of the calcareous shell of the serpulid Hydroides dianthus.- Journal of Structural Biology, San Diego, vol. 169, p. 145-160.
J.D. & W.J. (1969).- The influence of the periostracum on the shell structure of bivalve molluscs.- Calcified Tissue Research, New York, vol. 3, p. 274-283.
H. & A. (2004).- Introductory oceanography.- Pearson Prentice Hall, New Jersey, p. 151-152.
G.J. (1977).- The Mesozoic marine revolution; evidence from snails, predators and grazers.- Paleobiology, Tulsa, vol. 3, p. 245-258.
O. (2005).- The tube ultrastructure of serpulids (Annelida, Polychaeta) Pentaditrupa subtorquata, Cretaceous and Nogrobs cf. vertebralis, Jurassic from Germany.- Proceedings of the Estonian Academy of Sciences, Geology, Tallinn, vol. 54, p. 260-265.
O. (2006).- Tentaculitoid affinities of the tubeworm-like fossil Tymbochoos sinclairi (, 1937) from the Ordovician of North America.- Geobios, Paris, vol. 39, p. 739-742.
O. (2007).- Taxonomic implications and fossilization of tube ultrastructure of some Cenozoic serpulids (Annelida, Polychaeta) from Europe.- Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, Stuttgart, vol. 244, p. 115-128.
O. (2010).- Adaptive strategies in the evolution of encrusting tentaculitoid tubeworms.- Palæogeography, Palæoclimatology, Palæoecology, Amsterdam, vol. 292, p. 211-221.
O. (2011).- The role of an internal organic tube lining in the biomineralization of serpulid tubes.- Carnets de Géologie - Notebooks on Geology, Brest, Letter 2011/01 (CG2011_L01), 4 p.
O. & H. (2008).- Tube structure and ultrastructure of serpulids from the Jurassic of France and Switzerland, its evolutionary implications.- Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, Stuttgart, vol. 250, p. 129-135.
O., H.A. ten & H. (2008a).- On the tube ultrastructure and origin of calcification in sabellids (Annelida, Polychaeta).- Palaeontology, London, vol. 51, p. 295-301.
O., H.A. ten, H. & K. (2008b).- Ultrastructure and mineral composition of serpulid tubes (Polychaeta, Annelida).- Zoological Journal of the Linnean Society, London, vol. 154, p. 633-650.
O. & M. (2007).- The tentaculitid affinities of Anticalyptraea from the Silurian of Baltoscandia.- Palaeontology, London, vol. 50, p. 1385-1390.
O., M. & K. (2008c).- Microscopic evidence of serpulid affinities of the problematic fossil tube "Serpula" etalensis from the Lower Jurassic of Germany.- Lethaia, Oslo, vol. 41, p. 417-421.
O., K. & H.A. ten (2009).- Tube ultrastructure of Pomatoceros americanus (Polychaeta, Serpulidae): implications for the tube formation of serpulids.- Estonian Journal of Earth Sciences, Tallinn, vol. 58, p. 148-152.
O. & M.-A. (2008).- The earliest endosymbiotic mineralized tubeworms from the Silurian of Podolia, Ukraine.- Journal of Paleontology, Tulsa, vol. 82, p. 409-414.
O. & H. (2005).- Observations on the morphology, and affinities of cornulitids from the Ordovician of Anticosti island and the Silurian of Gotland.- Journal of Paleontology, Tulsa, vol. 79, p. 726-737.
O. & H. (2009).- Calcareous tubeworms of the Phanerozoic.- Estonian Journal of Earth Sciences, Tallinn, vol. 58, p. 286-296.
O., H., H.A. ten & K. (2008d).- Unique Mg-calcite skeletal ultrastructure in the tube of the serpulid polychaete Ditrupa.- Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, Stuttgart, vol. 248, P. 79-89.
J., M. & M. (1991).- Sites of biomineralization in the Polychaete Pomatoceros triqueter (Serpulidae) with comments on some other species.- Ophelia, Supplement, Stenstrup, vol. 5, p. 661-667.
M.J. (1994).- Tube microstructure of Recent and Jurassic serpulid polychaetes and the question of the Palaeozoic 'spirorbids'.- Acta Palaeontologica Polonica, Warsaw, vol. 39, p. 1-15.
A. & S. (1978).- Secretion and the ultrastructure of the periostracum of some terebratulide brachiopods.- Proceedings of the Royal Society of London, (Series B, Biological Sciences), vol. 202, p. 191-209.
| Species | Clade | Multilayered tube with densely and sparsely packed crystals/ type of structures | Dense outer layer present or whole tube made of dense structure/type of structure | Material studied |
||
| Apomatus globifer | B | No | IOP | No | 1 | |
| Bathyvermilia langerhansi | B | Yes | HAC/HAC | Yes | HAC | 2 |
| Chitinopoma serrula | B | No | IOP | No | 3 | |
| Chitinopomoides wilsoni | B | No | SIOP | No | 4 | |
| Crucigera websteri | A | Yes | SPHP/IOP/LF/SPHP | Yes | SPHP | 5 |
| Crucigera zygophora | A | Yes | SPHP/IOP/SIOP | Yes | 6 | |
| Ditrupa arietina | A | Yes | RRP/IOP | Yes | RRP | 7 |
| Ditrupa gracillima | A | Yes | RRP/IOP | Yes | RRP | 8 |
| Ditrupa strangulata | A | Yes | RRP/FH | Yes | RRP | 9 |
| Ficopomatus enigmaticus | A | No | IOP | No | 10 | |
| Ficopomatus uschakovi | A | No | IOP | No | 11 | |
| Filograna implexa | B | No | IOP | No | 12 | |
| Filogranella elatensis | B | No | IOP | No | 13 | |
| Filogranula gracilis | B | No | IOP | No | 14 | |
| Floriprotis sabiuraensis | A | No | IOP/LF/SLF | No | 15 | |
| Galeolaria hystrix | A | No | LF/SIOP | No | 16 | |
| Hyalopomatus marenzelleri | A | No | IOP | No | 17 | |
| Hyalopomatus madreporae | A | No | IOP | No | 18 | |
| Hydroides dianthus | A | Yes | SPHP/IOP/LF | Yes | SPHP | 19 |
| Hydroides norvegicus | A | Yes | SPHP/IOP | Yes | SPHP | 20 |
| Hydroides spongicola | A | No | SIOP | No | 21 | |
| Janita fimbriata | B | No | IOP | No | 22 | |
| Josephella marenzelleri | B | No | IOP | No | 23 | |
| Laminatubus alvini | A | Yes | HAC/IOP | Yes | HAC | 24 |
| Marifugia cavatica | A | Yes | IOP/IOP* | No* | 25 | |
| Metavermilia multicristata | B | No | IOP | No | 26 | |
| Neovermilia falcigera | A? | Yes | IOPL/IOP | Yes | IOPL | 27 |
| Neovermilia sphaeropomatus | A | No | LF | No | 28 | |
| Paraprotis pulchra | B | No | IOP | No | 29 | |
| Pentaditrupa subtorquata | ? | Yes | SPHP/FH | Yes | SPHP | 30 |
| Placostegus tridentatus | A | No** | SP | Yes | SP | 31 |
| Pomatostegus stellatus | B | No | HAC | No | 32 | |
| Protis arctica | B | No | IOP | No | 33 | |
| Protula diomedeae | B | No | SOIOP | No | 34 | |
| Pseudovermilia madracicola | B | No | SIOP | No | 35 | |
| Pseudovermilia occidentalis | B | No | IOP | No | 36 | |
| Pyrgopolon ctenactis | A | No | SOSIOP | No | 37 | |
| Rhodopsis pusilla | B | No | IOP | No | 38 | |
| Rotularia spirulaea | ? | Yes | HAC/LF | Yes | HAC | 39 |
| Salmacina incrustans | B | No | IOP | No | 40 | |
| Semivermilia crenata | B | No | IOP | No | 41 | |
| Serpula crenata | A | Yes | SP/IOP | Yes | SP | 42 |
| Serpula israelitica | A | No | LF | No | 43 | |
| Serpula vermicularis | A | No | LF | No | 44 | |
| Spiraserpula caribensis | A | Yes | SPHP/SIOP/SPHP | Yes | SPHP | 45 |
| Spirobranchus giganteus | A | No | OF/SIOP | No | 46 | |
| Spirobranchus triqueter | A | No | LF | No | 47 | |
| Spirobranchus kraussii | A | Yes | SPHP/IOP/LF/SIOP | Yes | SPHP | 48 |
| Vermiliopsis infundibulum | B | No | SIOP | No | 49 | |
| Vitreotubus digeronimoi | A | No** | SP | Yes | SP | 50 |
** Single layered species with tube walls made entirely of densely packed crystals.
Table 1: Serpulid species studied in this paper and their tube ultrastructures.
No Yes: absence or presence of "Multilayered tube with densely and sparsely packed crystals".
Isotropic structures: HAC - homogeneous angular crystal structure, FH - fine grained homogeneous structure, IOP - irregularly oriented prismatic structure, IOPL - irregularly oriented platy structure, RHC - rounded homogeneous structure, SIOP - spherulitic irregularly oriented prismatic structure. Semi-oriented structures: SOIOP - semi-ordered irregularly oriented prismatic structure, SOSIOP - semi-ordered spherulitic oriented prismatic structure.
Oriented prismatic structures: RRP- regularly ridged prismatic structure, SP - simple prismatic structure, SPHP - spherulitic prismatic structure.
Oriented complex structures: LF -lamello-fibrillar structure, OF - ordered fibrillar structure, SLF - spherulitic lamello-fibrillar structure. Tube layers are ordered from outside (left) to lumen (right).
1. Apomatus globifer. Recent. Locality: Kara Sea, 71°N, 64°E, depth 122 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.0105.01)
2. Bathyvermilia langerhansi. Recent. Locality: Madeira, Porto Santo, St.4.180, depth 3499 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.4041)
3. Chitinopoma serrula. Recent. Locality: Iceland, Snaefellsnes Peninsula, depth 30 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.5034)
4. Chitinopomoides wilsoni. Recent. Locality: Antarctica, USNM Acq.224443, depth 80 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3166)
5. Crucigera websteri. Recent. Locality: Surinam, depth 60 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3589)
6. Crucigera zygophora. Recent. Locality: Canoe Bay, Alaska, USA, depth 8 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3287)
7. Ditrupa arietina. Recent. Locality: Sweden, Tjärnö, depth 10 m. Deposited: Natural History Museum, University of Tartu
8. Ditrupa gracillima. Fossil. Locality: Australia, VIC, Rutledge Creek, Middle Miocene (Bairnsdalian) Rutledge Creek 9. Member, presumable palaeo water depth 10 m. Deposited: Zoological Museum Amsterdam.
9. Ditrupa strangulata. Fossil. Locality: France, Paris Basin, Belleu near Soissons, Eocene (Middle Lutetian). Deposited: Zoological Museum Amsterdam (ZMA V.Pol. 3115)
10. Ficopomatus enigmaticus. Recent. Locality: Lake of Tunis, depth 8 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3779)
11. Ficopomatus uschakovi. Recent. Locality: Thailand, Kong Prao, Koh-Chang Island, depth 1-2 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3647)
12. Filograna implexa. Recent. Locality: UK, Orkney Island, depth 22 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3767)
13. Filogranella elatensis. Recent. Locality: Japan, Sesoko Island, Okinawa, depth 10 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3911)
14. Filogranula gracilis. Recent. Cape Verde Islands, Boa Vista, depth 111 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.4047)
15. Floriprotis sabiuraensis. Recent. Locality: Shimoshima Island, Amakusa, Japan, depth 10 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3929)
16. Galeolaria hystrix. Recent. Locality: New Zealand, Queen Charlotte Sound, depth 1-2 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3576)
17. Hyalopomatus marenzelleri. Recent. Locality: Canary Islands, Lanzarote, depth 1030-1070 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.4522)
18. Hyalopomatus madreporae. Recent. Locality: Santa Maria di Leuca, Italy, depth 497-790 m.
19. Hydroides dianthus. Recent. Locality: USA, Anna Maria Island, FL, depth 2 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3661)
20. Hydroides norvegicus. Recent. Norway, Skjerstad (Saltenfjord), depth 164 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.0463).
21. Hydroides spongicola. Recent. Locality: Netherlands Antilles, Curaçao, depth 7 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3584)
22. Janita fimbriata. Recent. Locality: Canary Islands, Lanzarote, depth 88 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.4072)
23. Josephella marenzelleri. Recent. Locality: France, Marseille, depth unknown. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3030)
24. Laminatubus alvini. Recent. Locality: East Pacific Rise, 09°N, 104°W, depth 2509 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3512)
25. Marifugia cavatica. Recent. Locality: Hercegovina, Popovo Polje fresh water caves, depth 1 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3102)
26. Metavermilia multicristata. Recent. Locality: Seychelles, N. of d'Arros Island, depth 55 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.4300)
27. Neovermilia falcigera. Fossil. Locality: Italy, Calabria, Pleistocene, depth 1580 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3501b)
28. Neovermilia sphaeropomatus. Recent. Locality: New Zealand, Cape Saunders, depth 10 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3274)
29. Paraprotis pulchra. Recent. Locality: Japan, Kushimoto, depth 70 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3379)
30. Pentaditrupa subtorquata. Fossil. Locality: Upper Campanian, Cretaceous of Höver by Hannover, Germany. Deposited: Zoological Museum Amsterdam (ZMA V.Pol. 3705)
31. Placostegus tridentatus. Recent. Locality: Norway, Bergensfjord, depth unknown. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.1105)
32. Pomatostegus stellatus. Recent. Locality: Netherlands Antilles, Curaçao, depth 0.5 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.5170)
33. Protis arctica. Recent. Locality: NE of Iceland, depth 1802 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3833)
34. Protula diomedeae. Recent. Locality: USA, Florida, depth 73 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.4271)
35. Pseudovermilia madracicola. Recent. Locality: Netherlands Antilles, Curaçao, Salinja Fuik, depth 27 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3751)
36. Pseudovermilia occidentalis. Recent. Locality: Cape Verde Islands, St. Luzia, depth 10 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.4090)
37. Pyrgopolon ctenactis. Recent. Locality: Netherlands Antilles, Bonaire, depth 15 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.4969)
38. Rhodopsis pusilla. Recent. Locality: Reunion Island, depth 5 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3623)
39. Rotularia spirulaea. Fossil. Locality: Eocene, Doss Trento, Italy. Deposited: Natural History Museum Vienna (NHMW 2006z0233/0013)
40. Salmacina incrustans. Recent. Locality: Spain Costa Brava, depth 0.5 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3814)
41. Semivermilia crenata. Recent. Locality: France, Marseille, depth 1 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3045)
42. Serpula crenata. Recent. Locality: Indonesia, 411 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.1739)
43. Serpula israelitica. Recent. Locality: Cape Verde Islands, São Vicente, depth unknown. Deposited: Zoological Museum Amsterdam.
44. Serpula vermicularis. Recent. Locality: Ireland, Ardbear Lough, depth 20 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.3780)
45. Spiraserpula caribensis. Recent. Locality: Netherlands Antilles, Curaçao, depth 1 m. Deposited: Zoological Museum Amsterdam
46. Spirobranchus giganteus. Recent. Locality: Netherlands Antilles, Curaçao, depth 6 m. Deposited: Zoological Museum Amsterdam
47. Spirobranchus triqueter. Recent. Locality: Sweden, Tjärnö, depth 10 m. Deposited: Natural History Museum, University of Tartu.
48. Spirobranchus kraussii. Recent. Locality: Teluk Slawi, Indonesia, depth 0.5 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.4748)
49. Vermiliopsis infundibulum. Recent. Locality: Canary Islands, Lanzarote, depth 80 m. Deposited: Zoological Museum Amsterdam (ZMA V.Pol.4036)
50. Vitreotubus digeronimoi. Recent. Locality: Seychelles, Platte Island, Sta.795, depth 600 m. Zoological Museum Amsterdam (ZMA V.Pol.4308)
Click on thumbnail to enlarge the image.
Cliquer sur la vignette pour agrandir l'image.
Figure 1:
Pyrgopolon mellevillei (NHMW 2006z0233/0005) London Clay, Eocene, England. Polished cross section of the tube, treated with the solution for 5 minutes, showing outer dense coarse crystal layer. SPHP - spherulitic prismatic structure; IOP - irregularly
oriented prismatic structure.
Figure 2:
Pyrgopolon mellevillei (NHMW 2006z0233/0005) London Clay, Eocene, England. Polished cross section of the tube through the outer dense tube layer (spherulitic prismatic structure), treated with the solution for 5 minutes. Ext - exterior.
Figure 3:
Pyrgopolon mellevillei (NHMW 2006z0233/0005) London Clay, Eocene,
England. Polished cross section of the tube through the inner sparsely packed fine crystal tube layer (irregularly oriented prismatic structure), treated with the solution for 5 minutes.
Figure 4:
Laminatubus alvini, East Pacific Rise, Recent. Polished cross section of the tube, showing outer dense coarse crystal layer (HAC) and inner sparsely packed fine crystal layer (IOP). HAC - homogeneous angular crystal structure; IOP -
irregularly oriented prismatic structure.
Figure 5:
Neovermilia falcigera, Italy, Calabria, Pleistocene. Polished cross section of the tube through outer dense coarse crystal tube layer (irregularly oriented platy structure), treated with solution for 5 minutes.
Figure 6: Neovermilia falcigera, Italy, Calabria, Pleistocene. Polished cross section of the tube through inner sparsely packed fine crystal tube layer (irregularly oriented prismatic structure), treated with solution for 5 minutes.
Figure 7:
Marifugia cavatica, Hercegovina, Popovo Polje, Recent. Polished cross section through outer sparsely packed tube layer, treated with 1% acetic acid for 1 minute.
Figure 8:
Marifugia cavatica, Hercegovina, Popovo Polje, Recent. Polished cross section through inner dense tube layer, treated with 1% acetic acid for 1 minute.
Click on thumbnail to enlarge the image.
Cliquer sur la vignette pour agrandir l'image.
Figure 9 : Phylogenetic tree of serpulids (cladogram of maximum parsimony analysis; modified after & , 2010). Genera studied here indicated with colors. Bold = taxa with two or more tube layers. Red = taxa with outer dense tube layer. Yellow = outer sparsely packed fine crystal tube layer. P = oriented prismatic structures; HA = homogeneous angular crystal structure. Pomatoceros in meantime = Spirobranchus.