![]() In the Lower Aptian historical stratotype area (Cassis-La Bédoule, SE France), a geochemical study of the Gargasian (Middle Aptian) marl-limestone alternations of the La Marcouline quarry complements data already obtained from Bedoulian
(Early Aptian) sediments there. Nannoconids are the main carbonate producers in both limestones and
marls. Although diagenetic minerals, such as ankerite (2.5%) are present in small amounts, the
trace-element content of bulk carbonate is very close to that of Nannoconus
spp. so geochemical sequences can be defined. The long-term evolution of trace-element content was not affected by diagenetic processes, variations in carbonate mineralogy, or a change of carbonate producers. An increase of around 500 ppm in the strontium content of bulk carbonate occurs between the base of the Cabri zone
(late Bedoulian) and the Algerianus zone (late Gargasian). This evolution is linked to fluctuations in seawater Sr/Ca ratios caused by variability in the influx of hydrothermal and river waters, by changes in the ratio of aragonite/calcite production and by shifts in sea level. The eustatic sequence Aptian 4, its parasequences and its key surfaces (sequence boundaries, maximum flooding surface) are clearly reflected in the evolution of
the bulk-carbonate contents of manganese.
In the Lower Aptian historical stratotype area (Cassis-La Bédoule, SE France), a geochemical study of the Gargasian (Middle Aptian) marl-limestone alternations of the La Marcouline quarry complements data already obtained from Bedoulian
(Early Aptian) sediments there. Nannoconids are the main carbonate producers in both limestones and
marls. Although diagenetic minerals, such as ankerite (2.5%) are present in small amounts, the
trace-element content of bulk carbonate is very close to that of Nannoconus
spp. so geochemical sequences can be defined. The long-term evolution of trace-element content was not affected by diagenetic processes, variations in carbonate mineralogy, or a change of carbonate producers. An increase of around 500 ppm in the strontium content of bulk carbonate occurs between the base of the Cabri zone
(late Bedoulian) and the Algerianus zone (late Gargasian). This evolution is linked to fluctuations in seawater Sr/Ca ratios caused by variability in the influx of hydrothermal and river waters, by changes in the ratio of aragonite/calcite production and by shifts in sea level. The eustatic sequence Aptian 4, its parasequences and its key surfaces (sequence boundaries, maximum flooding surface) are clearly reflected in the evolution of
the bulk-carbonate contents of manganese.
![]() Aptian; Gargasian; pelagic carbonates; magnesium; strontium; manganese; iron; Nannoconus; seawater Sr/Ca; chemostratigraphy; sequence stratigraphy
Aptian; Gargasian; pelagic carbonates; magnesium; strontium; manganese; iron; Nannoconus; seawater Sr/Ca; chemostratigraphy; sequence stratigraphy
M. de, L., C., M. & G. (2007).- Fluctuations of sea-water chemistry during Gargasian (Middle Aptian) time. Data from trace-element content (Mg, Sr, Mn, Fe) in hemipelagic carbonates from La Marcouline Quarry (Cassis, SE France).- Carnets de Géologie / Notebooks on Geology, Brest, Article 2007/03 (CG2007_A03)
![]() Fluctuations
de la chimie de l'eau de mer au cours du Gargasien (Aptien Moyen). Apports des
teneurs en éléments traces (Mg, Sr, Mn et Fe) des carbonates hémipélagiques
de la carrière de La Marcouline (Cassis, Sud-Est France).- Dans la région du stratotype historique de l'Aptien inférieur (Cassis-La Bédoule, SE France), les sédiments alternants du Gargasien (Aptien supérieur) de la carrière de la Marcouline ont été étudiés du point de vue géochimique. Le dosage des éléments traces (Sr, Mg, Mn et Fe) du carbonate total a permis de compléter les données déjà obtenues sur le Bédoulien et de définir une zonation chimiostratigraphique du Gargasien. Les Nannoconus sont les producteurs carbonatés principaux aussi bien dans les bancs calcaires que dans les bancs marneux. Les différences géochimiques observées entre les marnes (enrichies en Sr et Mg) et les calcaires (enrichis en Mn et Fe) ne peuvent être réduites à une variation des producteurs ou à une diagenèse différentielle. En dépit de la présence de traces d'ankérite d'origine diagénétique
(2,5%), l'enregistrement des éléments-traces au sein du carbonate total reste très proche de celui des fractions pures en Nannoconus et permet de définir les séquences géochimiques. Dans la continuité du processus initié dans le Bédoulien supérieur (base de la zone à Cabri), les teneurs en strontium croissent régulièrement durant le Gargasien. Au total une augmentation de l'ordre de 500 ppm survient entre la base de la zone à Cabri (Bédoulien supérieur) et la zone à Algerianus (Gargasien supérieur). Elle traduit une variation du rapport Sr/Ca de l'eau de mer, à mettre en relation avec le bilan des apports hydrothermaux et fluviatiles, le rapport de la sédimentation aragonitique à la sédimentation calcitique et les variations du niveau marin. Les teneurs en manganèse ont permis de mettre en évidence la séquence eustatique Aptien 4 et les paraséquences qui la composent, ainsi que de localiser ses surfaces clefs (limites de séquence et surface d'inondation maximale).
Fluctuations
de la chimie de l'eau de mer au cours du Gargasien (Aptien Moyen). Apports des
teneurs en éléments traces (Mg, Sr, Mn et Fe) des carbonates hémipélagiques
de la carrière de La Marcouline (Cassis, Sud-Est France).- Dans la région du stratotype historique de l'Aptien inférieur (Cassis-La Bédoule, SE France), les sédiments alternants du Gargasien (Aptien supérieur) de la carrière de la Marcouline ont été étudiés du point de vue géochimique. Le dosage des éléments traces (Sr, Mg, Mn et Fe) du carbonate total a permis de compléter les données déjà obtenues sur le Bédoulien et de définir une zonation chimiostratigraphique du Gargasien. Les Nannoconus sont les producteurs carbonatés principaux aussi bien dans les bancs calcaires que dans les bancs marneux. Les différences géochimiques observées entre les marnes (enrichies en Sr et Mg) et les calcaires (enrichis en Mn et Fe) ne peuvent être réduites à une variation des producteurs ou à une diagenèse différentielle. En dépit de la présence de traces d'ankérite d'origine diagénétique
(2,5%), l'enregistrement des éléments-traces au sein du carbonate total reste très proche de celui des fractions pures en Nannoconus et permet de définir les séquences géochimiques. Dans la continuité du processus initié dans le Bédoulien supérieur (base de la zone à Cabri), les teneurs en strontium croissent régulièrement durant le Gargasien. Au total une augmentation de l'ordre de 500 ppm survient entre la base de la zone à Cabri (Bédoulien supérieur) et la zone à Algerianus (Gargasien supérieur). Elle traduit une variation du rapport Sr/Ca de l'eau de mer, à mettre en relation avec le bilan des apports hydrothermaux et fluviatiles, le rapport de la sédimentation aragonitique à la sédimentation calcitique et les variations du niveau marin. Les teneurs en manganèse ont permis de mettre en évidence la séquence eustatique Aptien 4 et les paraséquences qui la composent, ainsi que de localiser ses surfaces clefs (limites de séquence et surface d'inondation maximale).
![]() Aptien ; Gargasien ; carbonates pélagiques ; magnesium ; strontium ;
manganèse ; fer ; Nannoconus ; Sr/Ca de l'eau de mer ; chimiostratigraphie ; stratigraphie séquentielle
Aptien ; Gargasien ; carbonates pélagiques ; magnesium ; strontium ;
manganèse ; fer ; Nannoconus ; Sr/Ca de l'eau de mer ; chimiostratigraphie ; stratigraphie séquentielle
![]() &
(2004) and et alii
(2004) described the general setting of La Marcouline quarry, where the succession of Gargasian (Middle Aptian) beds is exposed continuously, and they specified its lithostratigraphic and biostratigraphic relationships to the Bedoulian (Lower Aptian) of the Cassis-La Bédoule area. Both
the marls and the limestones of the Gargasian beds of La Marcouline quarry were sampled and analyzed for stable carbon and oxygen isotopes (see & ,
2007) and for the trace elements in bulk carbonate (see results and discussion below). Samples were washed in distilled water, crushed and then dissolved in acetic acid (1N). Trace elements were analyzed by atomic absorption (Hitachi Z8100 spectrometer) using the method described by &
(1971; 1972) and
(1990). Analytical accuracy is around 5%. All data are listed in
Appendix 1
&
(2004) and et alii
(2004) described the general setting of La Marcouline quarry, where the succession of Gargasian (Middle Aptian) beds is exposed continuously, and they specified its lithostratigraphic and biostratigraphic relationships to the Bedoulian (Lower Aptian) of the Cassis-La Bédoule area. Both
the marls and the limestones of the Gargasian beds of La Marcouline quarry were sampled and analyzed for stable carbon and oxygen isotopes (see & ,
2007) and for the trace elements in bulk carbonate (see results and discussion below). Samples were washed in distilled water, crushed and then dissolved in acetic acid (1N). Trace elements were analyzed by atomic absorption (Hitachi Z8100 spectrometer) using the method described by &
(1971; 1972) and
(1990). Analytical accuracy is around 5%. All data are listed in
Appendix 1 ![]() . Gargasian sequences in La Marcouline section are defined geochemically and
labeled in continuity with the Bedoulian ones as proposed in the same geographic area by & de (1998).
. Gargasian sequences in La Marcouline section are defined geochemically and
labeled in continuity with the Bedoulian ones as proposed in the same geographic area by & de (1998).
![]() In La Marcouline quarry, Gargasian sedimentation is of a hemipelagic type in which marl and marly limestone deposits alternate regularly. Contacts between the two lithologies are transitional. Overall, the thickness of limestone beds relative to marly ones decreases upward
(Fig. 1
In La Marcouline quarry, Gargasian sedimentation is of a hemipelagic type in which marl and marly limestone deposits alternate regularly. Contacts between the two lithologies are transitional. Overall, the thickness of limestone beds relative to marly ones decreases upward
(Fig. 1 ![]() ). A parasequence pattern is superimposed on this general trend and there are nine parasequences of momentary thickening upward in limestone beds:
). A parasequence pattern is superimposed on this general trend and there are nine parasequences of momentary thickening upward in limestone beds:
![]() We note that the thickness of each parasequence increases upward throughout the observed Gargasian: Parasequence 2 occupies 2.5 m whereas parasequence 8 spans 11 m.
We note that the thickness of each parasequence increases upward throughout the observed Gargasian: Parasequence 2 occupies 2.5 m whereas parasequence 8 spans 11 m.
![]() The CaCO3 content of each bed is in accord with the marl/limestone alternations as delineated in the outcrop scale
(Fig. 1
The CaCO3 content of each bed is in accord with the marl/limestone alternations as delineated in the outcrop scale
(Fig. 1 ![]() ). The CaCO3 content of the limestone beds ranges from
64.4% to 87.5% (mean 77.6%) and the CaCO3 content of the marly beds from 53% to
77.4% (mean 62.8%). In both limestones and marls, the CaCO3 content decreases upward in the section (more markedly in parasequences 6 through 9). A comparison with Bedoulian data
(Fig. 2
). The CaCO3 content of the limestone beds ranges from
64.4% to 87.5% (mean 77.6%) and the CaCO3 content of the marly beds from 53% to
77.4% (mean 62.8%). In both limestones and marls, the CaCO3 content decreases upward in the section (more markedly in parasequences 6 through 9). A comparison with Bedoulian data
(Fig. 2 ![]() )
from the same area ( & de , 1998; et alii,
2005) shows that the CaCO3 content of the Gargasian beds at La Marcouline is intermediate between that of the strata of
early Bedoulian age (Kuznetsovae and Blowi foraminiferal zones) and that of the sequence of
late Bedoulian age (Cabri zone). CaCO3 content appear to be
more variable during the Gargasian than during the Bedoulian. This pattern is
caused by the fact that in the Bedoulian interval only limestones were sampled
whereas in the Gargasian both limestones and marls were collected.
)
from the same area ( & de , 1998; et alii,
2005) shows that the CaCO3 content of the Gargasian beds at La Marcouline is intermediate between that of the strata of
early Bedoulian age (Kuznetsovae and Blowi foraminiferal zones) and that of the sequence of
late Bedoulian age (Cabri zone). CaCO3 content appear to be
more variable during the Gargasian than during the Bedoulian. This pattern is
caused by the fact that in the Bedoulian interval only limestones were sampled
whereas in the Gargasian both limestones and marls were collected.
![]() The difference in the amount of CaCO3 in limestone and marl is not constant throughout the Gargasian succession
(Fig. 1
The difference in the amount of CaCO3 in limestone and marl is not constant throughout the Gargasian succession
(Fig. 1 ![]() ). The mean difference is about 15% but values range from 30.2% (between beds 25 and 26) to 0.4% (between beds 85 and 86). Sequences with a markedly greater amount of CaCO3 in limestone over that in marl (beds 4-5, 23 to 26, 53-54, 78-79) alternate with sequences in which the difference is slight (beds 7-8, 27 to 33, 39-40, 67-68 and 82 to 86). In these successions, the "hard beds", called "limestones" in outcrop, may have a CaCO3 content nearly the same as or even lower than the "soft beds" (i.e. "marls"). These fluctuations define 5 sequences that start with small differences in the CaCO3 content of the two rock types and are more or less related to the pattern of the stratonomic parasequences described above
(Fig. 1
). The mean difference is about 15% but values range from 30.2% (between beds 25 and 26) to 0.4% (between beds 85 and 86). Sequences with a markedly greater amount of CaCO3 in limestone over that in marl (beds 4-5, 23 to 26, 53-54, 78-79) alternate with sequences in which the difference is slight (beds 7-8, 27 to 33, 39-40, 67-68 and 82 to 86). In these successions, the "hard beds", called "limestones" in outcrop, may have a CaCO3 content nearly the same as or even lower than the "soft beds" (i.e. "marls"). These fluctuations define 5 sequences that start with small differences in the CaCO3 content of the two rock types and are more or less related to the pattern of the stratonomic parasequences described above
(Fig. 1 ![]() ):
):
![]() (2006) and &
(2007) show that these fluctuations in CaCO3 content have a -like frequency. This finding led to their interpretation as reflections of oscillations in the orbit of the earth that affect climate and in turn the rate and characteristics of sedimentation.
(2006) and et alii
(2007) have carried out a detailed sedimentological study focused on the interval spanning beds 29 to 32. In this interval the compositions of marl and limestone beds are very similar in terms of carbonate particles and type of producers
(Fig. 3
(2006) and &
(2007) show that these fluctuations in CaCO3 content have a -like frequency. This finding led to their interpretation as reflections of oscillations in the orbit of the earth that affect climate and in turn the rate and characteristics of sedimentation.
(2006) and et alii
(2007) have carried out a detailed sedimentological study focused on the interval spanning beds 29 to 32. In this interval the compositions of marl and limestone beds are very similar in terms of carbonate particles and type of producers
(Fig. 3 ![]() ). The carbonate fraction is composed of a heterogeneous assemblage of calcareous nannofossils made up of coccoliths (around 8%, mainly Watznauearia barnesae and few Rhagodiscus spp., Biscutum spp. and Zeugrhabdotus spp.), and nannoconids (around 20-25%), a small quantity of planktonic
foraminiferal debris, structureless carbonate particles, i.e. carbonate macrocrystals (around 8%), and calcitic microcrystals (around 60%).
). The carbonate fraction is composed of a heterogeneous assemblage of calcareous nannofossils made up of coccoliths (around 8%, mainly Watznauearia barnesae and few Rhagodiscus spp., Biscutum spp. and Zeugrhabdotus spp.), and nannoconids (around 20-25%), a small quantity of planktonic
foraminiferal debris, structureless carbonate particles, i.e. carbonate macrocrystals (around 8%), and calcitic microcrystals (around 60%).
![]() Carbonate macrocrystals (8.5% of the carbonate fraction) range in size from 12 to 5 µm and their mineralogic composition includes calcite (6%) and ankerite
(2.5%). et alii
(2005) described the same kind of macrocrystals in the Late Cretaceous pelagic sediments
("Marnes de Bidart") under the Bay of Biscay (SW
France). In both locations geochemical data suggest that these particles result
from early diagenetic processes
(see infra). If so, the effects of this diagenesis appear to be quite limited.
Carbonate macrocrystals (8.5% of the carbonate fraction) range in size from 12 to 5 µm and their mineralogic composition includes calcite (6%) and ankerite
(2.5%). et alii
(2005) described the same kind of macrocrystals in the Late Cretaceous pelagic sediments
("Marnes de Bidart") under the Bay of Biscay (SW
France). In both locations geochemical data suggest that these particles result
from early diagenetic processes
(see infra). If so, the effects of this diagenesis appear to be quite limited.
![]() Microcrystals are defined as calcitic particles (between < 3 and 5 µm) with no biological shapes or microstructures detectable by optical and electronic microscopy. They correspond to the "micarb" of &
(1983), (1992), et alii
(1994) and &
(2002). Oxygen and carbon isotope ratios and crystallographic data
(DTA/TG) show that La Marcouline micarb are mainly minute fragments of Nannoconus
spp. (, 2006). This shows that the carbonate fraction of La Marcouline sediments is
dominanted by biogenic particles (around 90%) and that in both marls and limestones, Nannoconus
spp. are the main carbonate producers (around 80% by volume).
Microcrystals are defined as calcitic particles (between < 3 and 5 µm) with no biological shapes or microstructures detectable by optical and electronic microscopy. They correspond to the "micarb" of &
(1983), (1992), et alii
(1994) and &
(2002). Oxygen and carbon isotope ratios and crystallographic data
(DTA/TG) show that La Marcouline micarb are mainly minute fragments of Nannoconus
spp. (, 2006). This shows that the carbonate fraction of La Marcouline sediments is
dominanted by biogenic particles (around 90%) and that in both marls and limestones, Nannoconus
spp. are the main carbonate producers (around 80% by volume).
![]() The non-carbonate portion of the samples is mainly clays and quartz. In both marls and limestones the clay fraction
consists of illite (31%) and kaolinite (29%). The lithological
contrasts between limestones and marls are caused by dilution cycles which
reflect periodic changes in the supply of non-carbonate material during uniform carbonate production (,
2006; et alii,
2007).
The non-carbonate portion of the samples is mainly clays and quartz. In both marls and limestones the clay fraction
consists of illite (31%) and kaolinite (29%). The lithological
contrasts between limestones and marls are caused by dilution cycles which
reflect periodic changes in the supply of non-carbonate material during uniform carbonate production (,
2006; et alii,
2007).
![]() At La Marcouline the magnesium content of the carbonates of Gargasian age is relatively higher
(3027-6479 ppm, mean: 4306 ppm) than that of Bedoulian strata in the same Cassis region (Fig. 4
At La Marcouline the magnesium content of the carbonates of Gargasian age is relatively higher
(3027-6479 ppm, mean: 4306 ppm) than that of Bedoulian strata in the same Cassis region (Fig. 4 ![]() ,
& de ,
1998). In these underlying beds the Mg contents ranges consistently from
2500 to 4500 ppm (mean = 3726 ppm), but in the sediments spanning the transition from the Deshayesi to the Furcata ammonite zones, a higher content
(5000 to 6300 ppm) is related to the late Bedoulian anoxic event OAE1a.
,
& de ,
1998). In these underlying beds the Mg contents ranges consistently from
2500 to 4500 ppm (mean = 3726 ppm), but in the sediments spanning the transition from the Deshayesi to the Furcata ammonite zones, a higher content
(5000 to 6300 ppm) is related to the late Bedoulian anoxic event OAE1a.
![]() Compared to
the geochemical data obtained for Bedoulian deposits ( & de ,
1998), the Gargasian samples show stronger
fluctuations in both Mg and carbonate content. These differences may be caused
by the different sampling procedures chosen. Indeed, the Bedoulian samples were
taken from limestone beds only whereas the Gargasian ones were collected equally from both
lithologies.
Compared to
the geochemical data obtained for Bedoulian deposits ( & de ,
1998), the Gargasian samples show stronger
fluctuations in both Mg and carbonate content. These differences may be caused
by the different sampling procedures chosen. Indeed, the Bedoulian samples were
taken from limestone beds only whereas the Gargasian ones were collected equally from both
lithologies.
![]() In a general way the evolution of the Mg content of the bulk carbonate is in phase with changes in lithology
(Fig. 5
In a general way the evolution of the Mg content of the bulk carbonate is in phase with changes in lithology
(Fig. 5 ![]() ): Marls have a higher contents (mean
4557 ppm, average 3027-6479 ppm) than limestones (mean 4039 ppm, average 3035-6046
ppm). Nevertheless, the long-term evolution of Mg content throughout the Gargasian succession may not be related to lithology alone. In the lower part of the sequences (beds 2 to 6, 22 to 26, 46 to 48, 57 to 60) many samples of both marl and limestone are low in magnesium while in the uppermost portion (from bed 64 to top) the content of magnesium in both marl and limestone increases in a similar manner.
): Marls have a higher contents (mean
4557 ppm, average 3027-6479 ppm) than limestones (mean 4039 ppm, average 3035-6046
ppm). Nevertheless, the long-term evolution of Mg content throughout the Gargasian succession may not be related to lithology alone. In the lower part of the sequences (beds 2 to 6, 22 to 26, 46 to 48, 57 to 60) many samples of both marl and limestone are low in magnesium while in the uppermost portion (from bed 64 to top) the content of magnesium in both marl and limestone increases in a similar manner.
![]() Throughout the Gargasian succession at La Marcouline there is an overall trend toward an increase in Mg contents from
3000-4000 ppm at the base to 5000-6000 ppm at the top
(Fig. 5
Throughout the Gargasian succession at La Marcouline there is an overall trend toward an increase in Mg contents from
3000-4000 ppm at the base to 5000-6000 ppm at the top
(Fig. 5 ![]() ). The Mg content increases in five positive excursions, each ended by a negative shift. The lowest samples figured are the uppermost portion of the last geochemical sequence described by
& de (1998) in Bedoulian outcrops. The first Gargasian Mg sequence runs from bed 3
(3313 ppm) to bed 22 (base of a well-characterized triplet in outcrop, 3113 ppm) and reaches its highest reading,
4818 ppm, in bed 17. This first sequence is attributed to the Cabri (Luterbacheri) and early Ferreolensis zones and is
labelled Garg.Mg1, in accordance with the numbering system of
& de (1998). In the second sequence, (Garg.Mg2), from bed 22 to 36
(3738 ppm), the Mg content reaches a maxinum of 4447 ppm in the upper part of bed 31. The third Mg sequence (Garg.Mg3) spans beds 36 to 46
(3098 ppm) with a maximum in bed 39 (5402 ppm). In the Garg.Mg4 sequence, Mg values rise to
5748 ppm in bed 46 and the upper boundary is again a negative shift, recorded in bed 58
(3206 ppm). The last well-exposed Mg sequence (Garg.Mg5) spans beds 58 to 86
(5041 ppm) with the highest Mg content (6046 ppm) in bed 82. At La Marcouline quarry, only the initial stages of the Garg.Mg6 sequence are accessible.
). The Mg content increases in five positive excursions, each ended by a negative shift. The lowest samples figured are the uppermost portion of the last geochemical sequence described by
& de (1998) in Bedoulian outcrops. The first Gargasian Mg sequence runs from bed 3
(3313 ppm) to bed 22 (base of a well-characterized triplet in outcrop, 3113 ppm) and reaches its highest reading,
4818 ppm, in bed 17. This first sequence is attributed to the Cabri (Luterbacheri) and early Ferreolensis zones and is
labelled Garg.Mg1, in accordance with the numbering system of
& de (1998). In the second sequence, (Garg.Mg2), from bed 22 to 36
(3738 ppm), the Mg content reaches a maxinum of 4447 ppm in the upper part of bed 31. The third Mg sequence (Garg.Mg3) spans beds 36 to 46
(3098 ppm) with a maximum in bed 39 (5402 ppm). In the Garg.Mg4 sequence, Mg values rise to
5748 ppm in bed 46 and the upper boundary is again a negative shift, recorded in bed 58
(3206 ppm). The last well-exposed Mg sequence (Garg.Mg5) spans beds 58 to 86
(5041 ppm) with the highest Mg content (6046 ppm) in bed 82. At La Marcouline quarry, only the initial stages of the Garg.Mg6 sequence are accessible.
![]() The increase in Sr in the sediments of the Bedoulian type locality
(Fig. 6
The increase in Sr in the sediments of the Bedoulian type locality
(Fig. 6 ![]() ,
& de ,
1998) from the base of the Cabri Zone upward continues throughout the La Marcouline Gargasian series, rising from 626 ppm in bed 1 to 896 ppm in bed 81.
,
& de ,
1998) from the base of the Cabri Zone upward continues throughout the La Marcouline Gargasian series, rising from 626 ppm in bed 1 to 896 ppm in bed 81.
![]() The
correlation of Sr content with variations in lithology is less obvious than it is for Mg
(Fig. 5
The
correlation of Sr content with variations in lithology is less obvious than it is for Mg
(Fig. 5 ![]() ). Nevertheless, in marls the content is slightly higher (mean 711 ppm, average 588–896 ppm) than in limestones (mean 699 ppm, average 590 – 845 ppm). The effects of lithology are clear only at and near the base of the outcrop (base to bed 22) where the difference in the Sr content of marls and limestones is on the order of 40/50 ppm. In the higher sequences the long-term increase in Sr content screens difference caused by changes in lithology.
). Nevertheless, in marls the content is slightly higher (mean 711 ppm, average 588–896 ppm) than in limestones (mean 699 ppm, average 590 – 845 ppm). The effects of lithology are clear only at and near the base of the outcrop (base to bed 22) where the difference in the Sr content of marls and limestones is on the order of 40/50 ppm. In the higher sequences the long-term increase in Sr content screens difference caused by changes in lithology.
![]() As for Mg, the overall increase in Sr upward is supplemented by positive excursions marking the geochemical sequences. The first
(Garg.Sr1) indicates an increase in Sr from 605 ppm (bed 3) to maxima of 732 ppm in beds 9 and 13
followed by a decrease to 588 ppm (bed 22). The negative shift in bed 22 (base of the outcrop triplet) seems to be a major geochemical break. The second positive excursion
(Garg.Sr2) extends from bed 23 to bed 36 (646 ppm) with a maximum in bed 31 (759 ppm). The highest value of
Garg.Sr3 is in bed 49 (758 ppm) and the sequence ends in bed 58 (681 ppm). We note that this sequence
covers two magnesium sequences (Garg.Mg3 and 4). The upper boundary of sequence
Garg.Sr4 is located at bed 86 (800 ppm). Its maximum content is recorded in bed 81 (896 ppm).
As for Mg, the overall increase in Sr upward is supplemented by positive excursions marking the geochemical sequences. The first
(Garg.Sr1) indicates an increase in Sr from 605 ppm (bed 3) to maxima of 732 ppm in beds 9 and 13
followed by a decrease to 588 ppm (bed 22). The negative shift in bed 22 (base of the outcrop triplet) seems to be a major geochemical break. The second positive excursion
(Garg.Sr2) extends from bed 23 to bed 36 (646 ppm) with a maximum in bed 31 (759 ppm). The highest value of
Garg.Sr3 is in bed 49 (758 ppm) and the sequence ends in bed 58 (681 ppm). We note that this sequence
covers two magnesium sequences (Garg.Mg3 and 4). The upper boundary of sequence
Garg.Sr4 is located at bed 86 (800 ppm). Its maximum content is recorded in bed 81 (896 ppm).
![]() The development of the Sr and
Mg profiles follow a similar course, so are easily comparable
(Fig. 7
The development of the Sr and
Mg profiles follow a similar course, so are easily comparable
(Fig. 7 ![]() ); the long-term trends both show an upward rise but lithology had a greater effect on Mg than on Sr.
Figure 4
); the long-term trends both show an upward rise but lithology had a greater effect on Mg than on Sr.
Figure 4 ![]() shows the correlation between these two trace elements. The correlation coefficient (r) is 0.69 for 96 samples and the equation of the correlation line is:
shows the correlation between these two trace elements. The correlation coefficient (r) is 0.69 for 96 samples and the equation of the correlation line is:
![]() [Sr] ppm = 0.074
[Mg] ppm + 381
[Sr] ppm = 0.074
[Mg] ppm + 381
![]() The overlap of the areas of marl and limestone data on
Figure 4
The overlap of the areas of marl and limestone data on
Figure 4 ![]() shows that the diagenesis of marls and limestones differs slightly, but may not play a major role in the quantification of Sr and Mg.
shows that the diagenesis of marls and limestones differs slightly, but may not play a major role in the quantification of Sr and Mg.
![]() The quantities of Mn in the bulk carbonates of the Gargasian strata of La Marcouline
(Fig. 8
The quantities of Mn in the bulk carbonates of the Gargasian strata of La Marcouline
(Fig. 8 ![]() , mean 285 ppm, average 175-399 ppm) are higher than those of Bedoulian age, excluding high values (up to 450 ppm) in beds at the base of the Upper Bedoulian (base of the Deshayesi ammonite
Zone) which are linked to an anoxic and methane hydrate event ( et alii,
2001; et alii,
2002;
& de , 1998;
et alii, 2005).
, mean 285 ppm, average 175-399 ppm) are higher than those of Bedoulian age, excluding high values (up to 450 ppm) in beds at the base of the Upper Bedoulian (base of the Deshayesi ammonite
Zone) which are linked to an anoxic and methane hydrate event ( et alii,
2001; et alii,
2002;
& de , 1998;
et alii, 2005).
![]() As noted above for Sr, lithology influences the quantity of Mn slightly
(Fig. 9
As noted above for Sr, lithology influences the quantity of Mn slightly
(Fig. 9 ![]() ) but its effect is contrary to that made on strontium: Limestones have a higher content of Mn than marls. The effect of lithology on Mn content is well expressed only in the first sequence of the La Marcouline series where a difference of 20 to 60 ppm is measured. In the other sequences, long-term increasing or decreasing trends tend to mask the role of lithology.
) but its effect is contrary to that made on strontium: Limestones have a higher content of Mn than marls. The effect of lithology on Mn content is well expressed only in the first sequence of the La Marcouline series where a difference of 20 to 60 ppm is measured. In the other sequences, long-term increasing or decreasing trends tend to mask the role of lithology.
![]() Three Mn geochemical sequences are defined. During the first (Garg.Mn1) the Mn content rises slightly, through its variations related to lithology, from bed 5 (229 ppm) to a maximum of 319 ppm and decreases to 227 ppm (bed 25-26). The second sequence (Garg.Mn2) presents a more obvious positive excursion (maximum: 381 ppm in beds 31-33). Its upper boundary is located in bed 47 (244 ppm). The third (Garg.Mn3) reaches a
maximum value in bed 62 (399 ppm) and ends in bed 81 or 83 (175 ppm). Low values (around 170-180 ppm) in the interval comprising beds 84 to 89 connote the beginning of the fourth sequence (Garg.Mn4).
Three Mn geochemical sequences are defined. During the first (Garg.Mn1) the Mn content rises slightly, through its variations related to lithology, from bed 5 (229 ppm) to a maximum of 319 ppm and decreases to 227 ppm (bed 25-26). The second sequence (Garg.Mn2) presents a more obvious positive excursion (maximum: 381 ppm in beds 31-33). Its upper boundary is located in bed 47 (244 ppm). The third (Garg.Mn3) reaches a
maximum value in bed 62 (399 ppm) and ends in bed 81 or 83 (175 ppm). Low values (around 170-180 ppm) in the interval comprising beds 84 to 89 connote the beginning of the fourth sequence (Garg.Mn4).
![]() The Gargasian portion of the La Marcouline series has a very high content of Fe. It increases from
1600 to 2000 ppm at the base to 2000-3000 ppm at the top. This increase is continuous with that of the acceleration of its trend in Bedoulian strata which began in sediments attributed to the base of the Cabri foraminiferal
Zone and becomes more prominent in the upper part of the Deshayesi ammonite
Zone. The sedimentological study on the interval comprising beds 29 to 32 (,
2006; et alii,
2007) has shown that an important part of the Fe content of bulk carbonates is related to the presence of ankerite macroparticles
(Fig. 3
The Gargasian portion of the La Marcouline series has a very high content of Fe. It increases from
1600 to 2000 ppm at the base to 2000-3000 ppm at the top. This increase is continuous with that of the acceleration of its trend in Bedoulian strata which began in sediments attributed to the base of the Cabri foraminiferal
Zone and becomes more prominent in the upper part of the Deshayesi ammonite
Zone. The sedimentological study on the interval comprising beds 29 to 32 (,
2006; et alii,
2007) has shown that an important part of the Fe content of bulk carbonates is related to the presence of ankerite macroparticles
(Fig. 3 ![]() ).
).
![]() The amount of Fe ranges widely in function of the lithology
(Fig. 9
The amount of Fe ranges widely in function of the lithology
(Fig. 9 ![]() ). Limestones have a greater amount of Fe than marls, a fact already noted for Mn. The difference ranges between 400 and
1000 ppm. Beds 30 to 33
(the interval in which the maxima of the Garg.Fe2 sequence is reached) are an exception
because marls are richer in Fe than limestones.
). Limestones have a greater amount of Fe than marls, a fact already noted for Mn. The difference ranges between 400 and
1000 ppm. Beds 30 to 33
(the interval in which the maxima of the Garg.Fe2 sequence is reached) are an exception
because marls are richer in Fe than limestones.
![]() Three Fe sequences are distinguished
(Fig. 9
Three Fe sequences are distinguished
(Fig. 9 ![]() ): The first (Garg.Fe1) records a decrease in contents from
2000 ppm (bed 1) to 1219 ppm (bed 23, the base of the characteristic triplet). The second (Garg.Fe2) shows a positive excursion with a maximum at the base of bed 33
(3179 ppm) and is bounded upward by bed 47 (1369 ppm). The last sequence (Garg.Fe3) shows an increase of Fe up to
3000 ppm in beds 82-86.
): The first (Garg.Fe1) records a decrease in contents from
2000 ppm (bed 1) to 1219 ppm (bed 23, the base of the characteristic triplet). The second (Garg.Fe2) shows a positive excursion with a maximum at the base of bed 33
(3179 ppm) and is bounded upward by bed 47 (1369 ppm). The last sequence (Garg.Fe3) shows an increase of Fe up to
3000 ppm in beds 82-86.
![]() The stratigraphic evolution of trace elements in the La Marcouline section
(Figs. 5
The stratigraphic evolution of trace elements in the La Marcouline section
(Figs. 5 ![]() and 9
and 9 ![]() ) and the frame provided by
these geochemical sequences (Fig.
11
) and the frame provided by
these geochemical sequences (Fig.
11 ![]() ) highlight five geochemical breaks, of which the first (G1) is the most important. It is manifested very well in the values of the four trace elements and corresponds to the characteristic limestone triplet visible in outcrop. There are some slight differences in the location of the
break for the various trace elements: Limestone bed 22 for Sr and Mg, limestone bed 24 for Mn and marl beds 23 to 26 for Fe. Note that the transition between CaCO3 zone 1 and zone 2 starts in bed 26. This important geochemical shift does not coincide precisely with any of the bio-events recorded recently by et alii
(2005). It corresponds only to the upper part of stratonomic sequence S3. No geochemical anomaly is associated with the boundary (bed 14) between the Luterbacheri and Ferreolensis foraminiferal zones which, nevertheless, is the location of stratonomy sequence boundary S2/S3.
) highlight five geochemical breaks, of which the first (G1) is the most important. It is manifested very well in the values of the four trace elements and corresponds to the characteristic limestone triplet visible in outcrop. There are some slight differences in the location of the
break for the various trace elements: Limestone bed 22 for Sr and Mg, limestone bed 24 for Mn and marl beds 23 to 26 for Fe. Note that the transition between CaCO3 zone 1 and zone 2 starts in bed 26. This important geochemical shift does not coincide precisely with any of the bio-events recorded recently by et alii
(2005). It corresponds only to the upper part of stratonomic sequence S3. No geochemical anomaly is associated with the boundary (bed 14) between the Luterbacheri and Ferreolensis foraminiferal zones which, nevertheless, is the location of stratonomy sequence boundary S2/S3.
![]() The second break (G2), located in bed 36, is less important and involves only Sr and Mg. Located in stratonomic sequence S5, this anomaly coincides with the Ferreolensis/Barri zonal boundary.
The second break (G2), located in bed 36, is less important and involves only Sr and Mg. Located in stratonomic sequence S5, this anomaly coincides with the Ferreolensis/Barri zonal boundary.
![]() The third break (G3) is of moderate importance; for Mg it is located in bed 46 and for Mn and Fe in bed 47. The fourth boundary (G4) is of little importance and is located in bed 58 (Sr and Mg). The last break (G5) is in beds 81-83 for Sr, Mg and Mn, and in beds 83-86 for Fe. This break is near (slightly later than) the transition between the Ferreolensis and Algerianus zones (bed 80).
The third break (G3) is of moderate importance; for Mg it is located in bed 46 and for Mn and Fe in bed 47. The fourth boundary (G4) is of little importance and is located in bed 58 (Sr and Mg). The last break (G5) is in beds 81-83 for Sr, Mg and Mn, and in beds 83-86 for Fe. This break is near (slightly later than) the transition between the Ferreolensis and Algerianus zones (bed 80).
![]() Following the pioneer work of
(1969) bulk carbonate strontium has been used as a classical proxy of seawater chemistry in both pelagic ( & ,
1981; et alii,
1982; , 1986; et alii,
1999) and in neritic realms ( et alii,
1971, 1978; ,
1981; , 1999). In neritic environments, carbonate Sr/Ca are correlated with sea water Sr/Ca and salinity ( & ,
1980; , 1985) but late diagenesis often masks the primary record (,
1978, 1983). For pelagic carbonates,
(1985, 1986) has shown that the effects of late diagenesis on pelagic carbonates is reduced, so the Sr content of bulk carbonate provides a reliable approximation of the Sr content of nannofossils. This view is confirmed by Sr isotope studies made by &
(1993) who consider that 80% of the geochemical signal of upper Cretaceous pelagic carbonate is of primary origin. On the other hand et alii
(1999) and et alii
(2006) consider that diagenesis obscured paleoceanographic signals in Lower Cretaceous sediments of the North Atlantic, because of the addition of diagenetic cements during burial compaction. In the La Marcouline Gargasian sediments, the use of granulometric separation (see et alii,
2007, for detail of the method) does not confirm this point of view. The Sr content of the 5 µm fraction, composed mainly of nannoconids (up to 80%), is very similar to that of bulk carbonate
(Fig. 12
Following the pioneer work of
(1969) bulk carbonate strontium has been used as a classical proxy of seawater chemistry in both pelagic ( & ,
1981; et alii,
1982; , 1986; et alii,
1999) and in neritic realms ( et alii,
1971, 1978; ,
1981; , 1999). In neritic environments, carbonate Sr/Ca are correlated with sea water Sr/Ca and salinity ( & ,
1980; , 1985) but late diagenesis often masks the primary record (,
1978, 1983). For pelagic carbonates,
(1985, 1986) has shown that the effects of late diagenesis on pelagic carbonates is reduced, so the Sr content of bulk carbonate provides a reliable approximation of the Sr content of nannofossils. This view is confirmed by Sr isotope studies made by &
(1993) who consider that 80% of the geochemical signal of upper Cretaceous pelagic carbonate is of primary origin. On the other hand et alii
(1999) and et alii
(2006) consider that diagenesis obscured paleoceanographic signals in Lower Cretaceous sediments of the North Atlantic, because of the addition of diagenetic cements during burial compaction. In the La Marcouline Gargasian sediments, the use of granulometric separation (see et alii,
2007, for detail of the method) does not confirm this point of view. The Sr content of the 5 µm fraction, composed mainly of nannoconids (up to 80%), is very similar to that of bulk carbonate
(Fig. 12 ![]() ). In Beds 29 to 32, the mean Sr content of bulk carbonate is 753 ppm (ranging between 650 and 924 ppm) and the mean Sr content of the Nannoconus spp. fraction is 743 ppm (range 669 to
927 ppm). In marls the Sr content of bulk carbonate
fluctuates between 710 and 924 ppm (mean: 772 ppm) and the Sr content of the nannoconid-rich fraction between 705 and 927 (mean: 772 ppm). In the limestones bulk Sr content fluctuates from 650 to 772 ppm (mean: 728 ppm) and the Sr content of nannoconids from 669 to 759 (mean: 694).
). In Beds 29 to 32, the mean Sr content of bulk carbonate is 753 ppm (ranging between 650 and 924 ppm) and the mean Sr content of the Nannoconus spp. fraction is 743 ppm (range 669 to
927 ppm). In marls the Sr content of bulk carbonate
fluctuates between 710 and 924 ppm (mean: 772 ppm) and the Sr content of the nannoconid-rich fraction between 705 and 927 (mean: 772 ppm). In the limestones bulk Sr content fluctuates from 650 to 772 ppm (mean: 728 ppm) and the Sr content of nannoconids from 669 to 759 (mean: 694).
![]() Trace-element content in carbonates is controlled by the following equation:
Trace-element content in carbonates is controlled by the following equation:
[A/Ca]crystal = K.[A/Ca]sea water
where A and Ca are the concentrations of the trace-element and calcium and K the apparent distribution coefficient. For Sr and Mg, the content of carbonate is mainly the outcome of a sensu stricto co-precipitation process (substitution of Ca in the carbonate lattice) but other processes such as inclusion or absorption are not excluded. So the apparent distribution coefficient may include various processes of co-precipitation.
![]() Three factors control the KSr value: Carbonate mineralogy, temperature and nature of the CaCO3 producer (vital effect) if the carbonate is biogenic. Carbonate mineralogy plays a major role. For example, at typical temperatures for sedimentary environments, the distribution coefficients of strontium are 1.1 for aragonite, 0.1 for calcite, 0.09 for magnesian calcite and 0.01 for dolomite (,
1972; et alii,
1979; ,
1985; , 2001).
Three factors control the KSr value: Carbonate mineralogy, temperature and nature of the CaCO3 producer (vital effect) if the carbonate is biogenic. Carbonate mineralogy plays a major role. For example, at typical temperatures for sedimentary environments, the distribution coefficients of strontium are 1.1 for aragonite, 0.1 for calcite, 0.09 for magnesian calcite and 0.01 for dolomite (,
1972; et alii,
1979; ,
1985; , 2001).
![]() In hemipelagic deposits such as the La Marcouline sediments, the mineralogy is mainly calcitic. Ankerite is not concentrated enough (< 3%, ,
2006) to affect Sr concentrations in bulk carbonate
(Fig. 12
In hemipelagic deposits such as the La Marcouline sediments, the mineralogy is mainly calcitic. Ankerite is not concentrated enough (< 3%, ,
2006) to affect Sr concentrations in bulk carbonate
(Fig. 12 ![]() ). As a result, the distribution of Sr in Bedoulian-Gargasian times cannot be explained by fluctuations in carbonate mineralogy.
). As a result, the distribution of Sr in Bedoulian-Gargasian times cannot be explained by fluctuations in carbonate mineralogy.
![]() Temperature plays a minor role in the incorporation of strontium into inorganic calcite. KSrcalcite fluctuates very little: From 0.12 at 40°C to 0.076 at 98°C ( et alii,
1964). For organic calcite the relationship to temperature works inversely from that for molluscs (see
, 2002; et alii,
2006) or sometime does not work at all (e.g. benthic foraminifera, et alii,
2005).
Temperature plays a minor role in the incorporation of strontium into inorganic calcite. KSrcalcite fluctuates very little: From 0.12 at 40°C to 0.076 at 98°C ( et alii,
1964). For organic calcite the relationship to temperature works inversely from that for molluscs (see
, 2002; et alii,
2006) or sometime does not work at all (e.g. benthic foraminifera, et alii,
2005).
![]() Although potentially temperature could have exerted a control during the deposition of La Marcouline sediments, a direct comparison with isotopic data shows that this factor had no significant effect. The Sr content
(Fig. 6
Although potentially temperature could have exerted a control during the deposition of La Marcouline sediments, a direct comparison with isotopic data shows that this factor had no significant effect. The Sr content
(Fig. 6 ![]() ) begins to increase in strata of
late Bedoulian age (earliest Cabri foraminiferal Zone) while δ18O is declining from around -1,75 ‰ to -2,25 ‰ ( et alii,
1998) (thus indicating in first approximation a rise in temperature of roughly 2°C). Sr
content keeps on increasing from the late Cabri
Zone until the end of the Gargasian while during the same time interval δ18O
records a rise up to -1.75% followed by a new phase of decrease reaching values around –2,20 ‰ ( & ,
2007). Thus it is obvious that the increase in Sr during the
late Bedoulian and the Gargasian cannot have been affected only by a thermic factor.
) begins to increase in strata of
late Bedoulian age (earliest Cabri foraminiferal Zone) while δ18O is declining from around -1,75 ‰ to -2,25 ‰ ( et alii,
1998) (thus indicating in first approximation a rise in temperature of roughly 2°C). Sr
content keeps on increasing from the late Cabri
Zone until the end of the Gargasian while during the same time interval δ18O
records a rise up to -1.75% followed by a new phase of decrease reaching values around –2,20 ‰ ( & ,
2007). Thus it is obvious that the increase in Sr during the
late Bedoulian and the Gargasian cannot have been affected only by a thermic factor.
![]() Vital effects may be important too. The best known case is molluscs's production of aragonite which in comparison to inorganic aragonite contains much less Sr (,
1969). In hemipelagic deposits the carbonate producers are limited to foraminifera and nannofossils, so the force of various vital effects should be reduced.
Vital effects may be important too. The best known case is molluscs's production of aragonite which in comparison to inorganic aragonite contains much less Sr (,
1969). In hemipelagic deposits the carbonate producers are limited to foraminifera and nannofossils, so the force of various vital effects should be reduced.
![]() (1986), using Tethyan outcrops and DSDP oceanic drilling holes, has shown that during the last 140 Ma the Sr content of pelagic carbonate may differ in amount by as much as 700 ppm. This was confirmed by a recent and more precise study ( & ,
2001), in which it was shown that carbonate Sr/Ca varies by up to 80% over both long and short periods of time. For example during the Late Berriasian and the Early Valanginian (,
1993; & ,
1993; & ,
2001) an important increase in the Sr content of pelagic bulk carbonates occurs in the Tethys (Vocontian Basin) and various DSDP sites between the Otopeta ([Sr] = 400 ppm) and the Verrucosum ([Sr] =
1100 ppm) ammonite zones. In the same way, during the Barremian an increase of 600 ppm (600 to
1200 ppm) occurs in the Hugii
Zone. Increases in Sr are of the same amplitude in the La Bédoule/La Marcouline sections with values of around 400 ppm in sediments of
Barremian, early Bedoulian and early late Bedoulian ages (Blowi Zone) and around 850 ppm in sediments of the Gargasian (Ferreolensis/ Algerianus zone boundary,
Fig. 6
(1986), using Tethyan outcrops and DSDP oceanic drilling holes, has shown that during the last 140 Ma the Sr content of pelagic carbonate may differ in amount by as much as 700 ppm. This was confirmed by a recent and more precise study ( & ,
2001), in which it was shown that carbonate Sr/Ca varies by up to 80% over both long and short periods of time. For example during the Late Berriasian and the Early Valanginian (,
1993; & ,
1993; & ,
2001) an important increase in the Sr content of pelagic bulk carbonates occurs in the Tethys (Vocontian Basin) and various DSDP sites between the Otopeta ([Sr] = 400 ppm) and the Verrucosum ([Sr] =
1100 ppm) ammonite zones. In the same way, during the Barremian an increase of 600 ppm (600 to
1200 ppm) occurs in the Hugii
Zone. Increases in Sr are of the same amplitude in the La Bédoule/La Marcouline sections with values of around 400 ppm in sediments of
Barremian, early Bedoulian and early late Bedoulian ages (Blowi Zone) and around 850 ppm in sediments of the Gargasian (Ferreolensis/ Algerianus zone boundary,
Fig. 6 ![]() ). This increase takes place in two steps: The first, which occurs in the Cabri
Zone, ends at a negative shift of around 100 ppm at the Cabri/Ferreolensis boundary. The second spans the Ferreolensis and Algerianus zones.
). This increase takes place in two steps: The first, which occurs in the Cabri
Zone, ends at a negative shift of around 100 ppm at the Cabri/Ferreolensis boundary. The second spans the Ferreolensis and Algerianus zones.
![]() Such variations may reflect either changes in the Sr/Ca content of seawater over time, or variations in Sr partitioning in biogenic carbonates.
(1986) proposed that long-term fluctuations in seawater Sr/Ca are related to mid-ocean ridge activity and the hydrothermal budget of the ocean, while short-term variations are related to changes in sea-level, platform development (drowning vs emersion) and neritic carbonate mineralogy. &
(2002) insisted on this point by taking into account the alternation of calcite and aragonite periods in seawater (,
1975; & ,
1981; et alii,
1997; & ,
1998; , 2006). As the Sr partitioning coefficient is very different in calcite (KSrcalcite = 0.1) and aragonite (KSraragonite = 1), the ratio of aragonite to calcite production is important in the control of the Sr/Ca of seawater. This occurs through fluctuations in the amount of aragonite-rich neritic sedimentation (dependent on sea level) as well as the chemistry of seawater (the rate of precipitation of calcite changes in relation to the quantity of Mg).
Such variations may reflect either changes in the Sr/Ca content of seawater over time, or variations in Sr partitioning in biogenic carbonates.
(1986) proposed that long-term fluctuations in seawater Sr/Ca are related to mid-ocean ridge activity and the hydrothermal budget of the ocean, while short-term variations are related to changes in sea-level, platform development (drowning vs emersion) and neritic carbonate mineralogy. &
(2002) insisted on this point by taking into account the alternation of calcite and aragonite periods in seawater (,
1975; & ,
1981; et alii,
1997; & ,
1998; , 2006). As the Sr partitioning coefficient is very different in calcite (KSrcalcite = 0.1) and aragonite (KSraragonite = 1), the ratio of aragonite to calcite production is important in the control of the Sr/Ca of seawater. This occurs through fluctuations in the amount of aragonite-rich neritic sedimentation (dependent on sea level) as well as the chemistry of seawater (the rate of precipitation of calcite changes in relation to the quantity of Mg).
![]() Nevertheless variations through time of Sr partitioning in biogenic carbonate cannot be excluded ( & ,
2001; et alii,
2004; , 2005). Experimental coccolithophorid cultures have shown that the Sr partitioning coefficient is higher (DSrcalcite = 0.39, et alii,
2006) for this biogenic calcite than for inorganic precipitation. These new data confirm the previous work of
(1985): Analysis of modern coccolith oozes and sea water yielded 0.25 ≤ KSrcalcite ≤ 0.28 for ooze, versus 0.148 for inorganic calcite at the temperature of the Eastern Mediterranean Sea.
Nevertheless variations through time of Sr partitioning in biogenic carbonate cannot be excluded ( & ,
2001; et alii,
2004; , 2005). Experimental coccolithophorid cultures have shown that the Sr partitioning coefficient is higher (DSrcalcite = 0.39, et alii,
2006) for this biogenic calcite than for inorganic precipitation. These new data confirm the previous work of
(1985): Analysis of modern coccolith oozes and sea water yielded 0.25 ≤ KSrcalcite ≤ 0.28 for ooze, versus 0.148 for inorganic calcite at the temperature of the Eastern Mediterranean Sea.
![]() If nannoconid populations increased during Aptian time, the Sr content of bulk carbonate may have increased as well. Moreover, the nannofossil Sr coefficient appears to correlate
with growth and calcification rates ( et alii,
2002; , 2003; & ,
2003; et alii,
2004; et alii, 2005; et alii,
2005). Thus carbonate Sr/Ca measurements may provide valuable information on changes in the productivity of calcareous nannoplankton through time. But population growth does not explain the long-term increase in Sr at La Marcouline for the δ13C record shows that pelagic productivity regularly decreased through the
early-middle Gargasian (see & ,
2007).
If nannoconid populations increased during Aptian time, the Sr content of bulk carbonate may have increased as well. Moreover, the nannofossil Sr coefficient appears to correlate
with growth and calcification rates ( et alii,
2002; , 2003; & ,
2003; et alii,
2004; et alii, 2005; et alii,
2005). Thus carbonate Sr/Ca measurements may provide valuable information on changes in the productivity of calcareous nannoplankton through time. But population growth does not explain the long-term increase in Sr at La Marcouline for the δ13C record shows that pelagic productivity regularly decreased through the
early-middle Gargasian (see & ,
2007).
![]() An important part of the increase in Sr content in the
late Bedoulian and the Gargasian appears to be linked to an increase in the
Sr/Ca ratio of the seawater. A consensus exists concerning a molar Sr/Ca ratio value of 0.86 ± 0.04 x 10-2 in normal modern seawater (,
1969). However, (1985) has shown that this ratio may be more variable in relation to salinity and the nature of the adjacent continent (limestones vs silicates). Values of 0.938 ±
0.04*10-2 are proposed for the surface seawater of the western English Channel (mean chlorinity = 19.49 ‰); 0.796 ±
0.04*10-2 for the Eastern Mediterranean Sea (at a depth of –200m, mean chlorinity = 21.636 ‰) and 0.809 ±
0.03*10-2 for the Western Mediterranean Sea (from surface to –2085 m; mean chlorinity = 21.303 ‰). The Sr/Ca ratio of seawater does not vary in an important way with depth; nevertheless, in numerous sites Sr content was seen to increase between –500 and –800m because of the dissolution of
Acantharia tests (celestine).
An important part of the increase in Sr content in the
late Bedoulian and the Gargasian appears to be linked to an increase in the
Sr/Ca ratio of the seawater. A consensus exists concerning a molar Sr/Ca ratio value of 0.86 ± 0.04 x 10-2 in normal modern seawater (,
1969). However, (1985) has shown that this ratio may be more variable in relation to salinity and the nature of the adjacent continent (limestones vs silicates). Values of 0.938 ±
0.04*10-2 are proposed for the surface seawater of the western English Channel (mean chlorinity = 19.49 ‰); 0.796 ±
0.04*10-2 for the Eastern Mediterranean Sea (at a depth of –200m, mean chlorinity = 21.636 ‰) and 0.809 ±
0.03*10-2 for the Western Mediterranean Sea (from surface to –2085 m; mean chlorinity = 21.303 ‰). The Sr/Ca ratio of seawater does not vary in an important way with depth; nevertheless, in numerous sites Sr content was seen to increase between –500 and –800m because of the dissolution of
Acantharia tests (celestine).
![]() If we consider the data obtained from the Nannoconus
spp. fractions of beds 29 to 32, the mean Sr/Ca of Aptian calcite is around
0.872*10-2 (0.787 to 0.967*10-2). The use of the inorganic calcite KSr value determines an Aptian seawater Sr/Ca of around
0.793*10-2 which is consistent with the modern value. The use of KSrcalcite pelagic ooze of
(1986) or of KSrcoccoliths of et alii
(2006) leads to a very low Sr/Ca ratio (0.329*10-2 or
0.224*10-2) compared to the modern value. Due to the absence of Nannoconus
spp. in the modern ocean we cannot choose between these various options.
If we consider the data obtained from the Nannoconus
spp. fractions of beds 29 to 32, the mean Sr/Ca of Aptian calcite is around
0.872*10-2 (0.787 to 0.967*10-2). The use of the inorganic calcite KSr value determines an Aptian seawater Sr/Ca of around
0.793*10-2 which is consistent with the modern value. The use of KSrcalcite pelagic ooze of
(1986) or of KSrcoccoliths of et alii
(2006) leads to a very low Sr/Ca ratio (0.329*10-2 or
0.224*10-2) compared to the modern value. Due to the absence of Nannoconus
spp. in the modern ocean we cannot choose between these various options.
![]() Although the absolute value of Gargasian seawater Sr/Ca is not determinable, it is probable that this ratio increased
by 40–50% during the time between the early Cabri
Zone and the Algerianus Zone. This confirms the results of &
(2002) obtained through the analysis of brachiopods and rudists. On a geological time scale, four processes can be invoked to explain the increase of the Sr content of bulk carbonate during
late Bedoulian and Gargasian times:
Although the absolute value of Gargasian seawater Sr/Ca is not determinable, it is probable that this ratio increased
by 40–50% during the time between the early Cabri
Zone and the Algerianus Zone. This confirms the results of &
(2002) obtained through the analysis of brachiopods and rudists. On a geological time scale, four processes can be invoked to explain the increase of the Sr content of bulk carbonate during
late Bedoulian and Gargasian times:
![]() The second process does not seem realistic as the growth of Tethyan platforms ( & ,
1981; et alii, 1993; et alii,
1998) is not coincident, at this scale of frequency, with the Aptian long-term Sr fluctuations. Process 4 does not appear to play a significant role during this period, for nannoconids were the main pelagic producer of carbonate.
The second process does not seem realistic as the growth of Tethyan platforms ( & ,
1981; et alii, 1993; et alii,
1998) is not coincident, at this scale of frequency, with the Aptian long-term Sr fluctuations. Process 4 does not appear to play a significant role during this period, for nannoconids were the main pelagic producer of carbonate.
![]() During Aptian-Albian times the 87Sr/86Sr curve shows a negative excursion related to sea-floor hydrothermal activity ( & ,
2002). Consequently, we think that the increase in the Sr content of
late Bedoulian/Gargasian carbonates is due to an increase in the supply of Sr from hydrothermal sources and the development of conditions favoring the formation of calcite,
thus indicating a limited production of aragonite on the shelves ( & ,
1998; & ,
2002).
During Aptian-Albian times the 87Sr/86Sr curve shows a negative excursion related to sea-floor hydrothermal activity ( & ,
2002). Consequently, we think that the increase in the Sr content of
late Bedoulian/Gargasian carbonates is due to an increase in the supply of Sr from hydrothermal sources and the development of conditions favoring the formation of calcite,
thus indicating a limited production of aragonite on the shelves ( & ,
1998; & ,
2002).
![]() The difference in the Sr content of marls and limestones may be due to a change in the Sr incorporation coefficient of Nannoconus as it relates to growth and calcification rate ( et alii,
2002; , 2003; & ,
2003; et alii,
2004; et alii, 2005; et alii,
2005). This hypothesis of a higher KSrNannoconus, that causes a greater productivity during the deposition of marls, may be congruent with the long-term carbon isotope data (δ13C higher in
marls, & ,
2007) and with short-term data (,
2006; et alii,
2007).
The difference in the Sr content of marls and limestones may be due to a change in the Sr incorporation coefficient of Nannoconus as it relates to growth and calcification rate ( et alii,
2002; , 2003; & ,
2003; et alii,
2004; et alii, 2005; et alii,
2005). This hypothesis of a higher KSrNannoconus, that causes a greater productivity during the deposition of marls, may be congruent with the long-term carbon isotope data (δ13C higher in
marls, & ,
2007) and with short-term data (,
2006; et alii,
2007).
![]() The presence of traces of an Mg and Fe-rich mineral (ankerite,
Fig. 3
The presence of traces of an Mg and Fe-rich mineral (ankerite,
Fig. 3 ![]() ) introduces an important bias and renders impossible the use of bulk carbonate Fe and Mg content to define the chemical conditions of the La Marcouline sedimentary environment. This does not mean that the presence of this early diagenetic mineral is not influenced by the environment, but the link is not clear and the Mg and Fe content of the bulk carbonate is mainly a guide to the percentage of ankerite in the sediment.
) introduces an important bias and renders impossible the use of bulk carbonate Fe and Mg content to define the chemical conditions of the La Marcouline sedimentary environment. This does not mean that the presence of this early diagenetic mineral is not influenced by the environment, but the link is not clear and the Mg and Fe content of the bulk carbonate is mainly a guide to the percentage of ankerite in the sediment.
![]() A comparison of the Mg and Fe
contents of the separated granulometric fractions obtained from the samples of beds
29 to 32 shows that the fraction 12 µm, in which ankerite macroparticles are concentrated, has an Mg content of
10000–15000
ppm (Fig. 13
A comparison of the Mg and Fe
contents of the separated granulometric fractions obtained from the samples of beds
29 to 32 shows that the fraction 12 µm, in which ankerite macroparticles are concentrated, has an Mg content of
10000–15000
ppm (Fig. 13 ![]() ,
,
2006). The Mg content of fractions 20, 8 and 5 µm and bulk carbonate is lower but has a broad range like that of the fraction 12 µm. These observations bring out the correlation between the amount of ankerite and the Mg-Fe content. Only fine fractions (3 and < 3 µm) seem to be more or less ankerite free.
,
,
2006). The Mg content of fractions 20, 8 and 5 µm and bulk carbonate is lower but has a broad range like that of the fraction 12 µm. These observations bring out the correlation between the amount of ankerite and the Mg-Fe content. Only fine fractions (3 and < 3 µm) seem to be more or less ankerite free.
![]() Since et alii
(2005) described the same kind of ankerite crystals in
Late Cretaceous pelagic sediments, the occurrence of this mineral phase seems more common than was thought previously and in the pelagic realm limits the potential use of Mg as a paleotemperature tool.
Since et alii
(2005) described the same kind of ankerite crystals in
Late Cretaceous pelagic sediments, the occurrence of this mineral phase seems more common than was thought previously and in the pelagic realm limits the potential use of Mg as a paleotemperature tool.
![]() In spite of the presence of ankerite in Gargasian sediments, a surprisingly high degree of correlation exits between their Mg and Sr content
(Figs. 5
In spite of the presence of ankerite in Gargasian sediments, a surprisingly high degree of correlation exits between their Mg and Sr content
(Figs. 5 ![]() and 7
and 7 ![]() ). As ankerite is an important carrier of Mg and not of Sr, this means that the existence of long-term coeval Sr and Mg fluctuations in the Aptian marine environment in no way depended on the existence of this product of early diagenesis.
). As ankerite is an important carrier of Mg and not of Sr, this means that the existence of long-term coeval Sr and Mg fluctuations in the Aptian marine environment in no way depended on the existence of this product of early diagenesis.
![]() The same can be said for the larger part of the fluctuations of iron and Mn (even ankerite may contain traces of Mn). From the base of the measured section to bed 62-63, the plotted traces of Fe and Mn fluctuate more or less in phase, but in the upper part of the section they fluctuate in opposite directions: Fe content rises as Mn content decreases. As ankerite commonly forms by diagenesis under early redox conditions, a change in the redox environment may have occurred during late Ferreolensis
Zone times (this hypothesis is consistent with a general decrease of δ13C during the Gargasian ( & ,
2007)).
The same can be said for the larger part of the fluctuations of iron and Mn (even ankerite may contain traces of Mn). From the base of the measured section to bed 62-63, the plotted traces of Fe and Mn fluctuate more or less in phase, but in the upper part of the section they fluctuate in opposite directions: Fe content rises as Mn content decreases. As ankerite commonly forms by diagenesis under early redox conditions, a change in the redox environment may have occurred during late Ferreolensis
Zone times (this hypothesis is consistent with a general decrease of δ13C during the Gargasian ( & ,
2007)).
![]() Numerous studies (,
1983; et alii,
1989, 1993; et alii,
2000) led to the suggestion that the Mn content of pelagic bulk carbonates varies in response to a change in sea level.
(1993), &
(1993), de
(2000) and de et alii
(2000, 2001) have established geochemical criteria for the identification of systems tracts and sea-level changes from an analysis of sediments in the Vocontian Trough (SE France): Sequence boundaries are marked by a minimum in Mn content. That content increases in transgressive systems and reaches a maximum at the level of maximum flooding surfaces (mfs). Highstand systems have a lower amount of Mn. et alii
(2001) used this method to identify sequence boundaries in upper Cretaceous chalks on the Isle of Wight (England).
Numerous studies (,
1983; et alii,
1989, 1993; et alii,
2000) led to the suggestion that the Mn content of pelagic bulk carbonates varies in response to a change in sea level.
(1993), &
(1993), de
(2000) and de et alii
(2000, 2001) have established geochemical criteria for the identification of systems tracts and sea-level changes from an analysis of sediments in the Vocontian Trough (SE France): Sequence boundaries are marked by a minimum in Mn content. That content increases in transgressive systems and reaches a maximum at the level of maximum flooding surfaces (mfs). Highstand systems have a lower amount of Mn. et alii
(2001) used this method to identify sequence boundaries in upper Cretaceous chalks on the Isle of Wight (England).
![]() Using these geochemical criteria, three eustatic sequences are recognized in La Marcouline section. They correspond with the Gargasian Mn sequences 1 to 3
(Fig. 9
Using these geochemical criteria, three eustatic sequences are recognized in La Marcouline section. They correspond with the Gargasian Mn sequences 1 to 3
(Fig. 9 ![]() ). The candidate for the maximum flooding surface of the first eustatic sequence may be Bed 20 (high Mn content). Bed 14 (Luterbacheri/Ferreolensis zone boundary) may indicate a transgressive surface (increase of Mn content). The lower limit of the second sequence (lower Mn content) is in the upper part of the characteristic triplet (Bed 25-26). Its mfs is in the marly part of the series between bed 32 and the base of bed 33; the highstand systems tract includes two parasequences characterized by Mn fluctuations and ends in bed 47. The mfs of the third eustatic sequence is in bed 62 and ends in bed 82, in the lowest part of the Algerianus
Zone.
). The candidate for the maximum flooding surface of the first eustatic sequence may be Bed 20 (high Mn content). Bed 14 (Luterbacheri/Ferreolensis zone boundary) may indicate a transgressive surface (increase of Mn content). The lower limit of the second sequence (lower Mn content) is in the upper part of the characteristic triplet (Bed 25-26). Its mfs is in the marly part of the series between bed 32 and the base of bed 33; the highstand systems tract includes two parasequences characterized by Mn fluctuations and ends in bed 47. The mfs of the third eustatic sequence is in bed 62 and ends in bed 82, in the lowest part of the Algerianus
Zone.
![]() Although these sequences deduced from Mn fluctuations are clearly expressed, it is difficult to determine to which order of sea-level variation they correspond. The chronostratigraphic chart of et alii
(1998) has only one third-order sequence sensu (Ap. 4) during the time span of the Ferreolensis/ Algerianus zones
(Fig. 14
Although these sequences deduced from Mn fluctuations are clearly expressed, it is difficult to determine to which order of sea-level variation they correspond. The chronostratigraphic chart of et alii
(1998) has only one third-order sequence sensu (Ap. 4) during the time span of the Ferreolensis/ Algerianus zones
(Fig. 14 ![]() ). Thus two possibilities exist: Either the Gargasian Mn sequences are fourth-order sea-level parasequences of the Ap.4 third-order sequence, or one Middle Aptian third-order sequence is missing in the chart.
). Thus two possibilities exist: Either the Gargasian Mn sequences are fourth-order sea-level parasequences of the Ap.4 third-order sequence, or one Middle Aptian third-order sequence is missing in the chart.
![]() The question is: Which of these two possibilities is the correct one? It can be answered by integrating the evolution of the Mn content of the Gargasian in that of the long-term evolution of Mn at La Bédoule - La Marcouline
(Figs. 8
The question is: Which of these two possibilities is the correct one? It can be answered by integrating the evolution of the Mn content of the Gargasian in that of the long-term evolution of Mn at La Bédoule - La Marcouline
(Figs. 8 ![]() and 15
and 15 ![]() ). The positive excursions of Mn during the Gargasian clearly appear as parasequences in a third-order sequence that starts in the upper part of the Cabri
Zone and ends at the lower boundary of the Algerianus Zone. So this third-order sequence, including its three parasequences, seems to represent the fourth Aptian sequence in the chart of et alii
(1998). The maximum flooding surface may be located in beds 32-33, or in bed 62. The latter seems to be the better candidate when the long-term evolution of Mn values is the criterion
(Fig. 14
). The positive excursions of Mn during the Gargasian clearly appear as parasequences in a third-order sequence that starts in the upper part of the Cabri
Zone and ends at the lower boundary of the Algerianus Zone. So this third-order sequence, including its three parasequences, seems to represent the fourth Aptian sequence in the chart of et alii
(1998). The maximum flooding surface may be located in beds 32-33, or in bed 62. The latter seems to be the better candidate when the long-term evolution of Mn values is the criterion
(Fig. 14 ![]() ).
).
![]() However, the limits of our scheme, based on geochemistry, are not precisely coincident with those of et alii chart
(1998). In this chart, the fourth Aptian sequence begins in the uppermost part of the Cabri
Zone, just below the Cabri/Ferreolensis boundary and ends in the upper Algerianus
Zone. From a geochemical point of view, the fourth Aptian sequence starts in the middle of the Cabri
Zone and ends at the boundary between the Ferreolensis and Algerianus zones. Such discrepancies might also be a result of the fact that the zonal boundaries of the chart of et alii
(1998) were defined on the basis of taxonomic and biostratigraphic concepts which differ slightly from those of et alii
(2002, 2005).
However, the limits of our scheme, based on geochemistry, are not precisely coincident with those of et alii chart
(1998). In this chart, the fourth Aptian sequence begins in the uppermost part of the Cabri
Zone, just below the Cabri/Ferreolensis boundary and ends in the upper Algerianus
Zone. From a geochemical point of view, the fourth Aptian sequence starts in the middle of the Cabri
Zone and ends at the boundary between the Ferreolensis and Algerianus zones. Such discrepancies might also be a result of the fact that the zonal boundaries of the chart of et alii
(1998) were defined on the basis of taxonomic and biostratigraphic concepts which differ slightly from those of et alii
(2002, 2005).
![]() Nevertheless the succession in the La Marcouline outcrop does not preclude the presence of other highstand parasequences in the time represented by the Algerianus
Zone. To locate and to define precisely the limits of the Aptian 5 sequence complementary
upper Gargasian outcrops must be selected to carry out further Mn analyses.
Nevertheless the succession in the La Marcouline outcrop does not preclude the presence of other highstand parasequences in the time represented by the Algerianus
Zone. To locate and to define precisely the limits of the Aptian 5 sequence complementary
upper Gargasian outcrops must be selected to carry out further Mn analyses.
![]() This geochemical study of the Gargasian beds of La Marcouline quarry (Cassis-La Bédoule, SE France) complements data previously obtained from the Bedoulian historical stratotype area and leads to a comprehensive knowledge of geochemical fluctuations during the Early and Middle Aptian. Nannoconids are the main carbonate producers in both limestones and marls. Because of their low content of diagenetic minerals (such as ankerite), the trace-element records of the bulk carbonate closely approach those of Nannoconus
spp. so geochemical sequences can be defined. The long-term evolution of Sr and Mn content is linked to fluctuations in the chemistry of sea-water due to differences in the rate of their supply by rivers and hydrothermal sources, to changes in sea level and to variation in the ratio of aragonite to calcite production on the platforms. Diagenetic processes are not involved, nor are variations in carbonate mineralogy or changes in the number or nature of pelagic carbonate producers in the hemipelagic sediments of La Marcouline quarry.
This geochemical study of the Gargasian beds of La Marcouline quarry (Cassis-La Bédoule, SE France) complements data previously obtained from the Bedoulian historical stratotype area and leads to a comprehensive knowledge of geochemical fluctuations during the Early and Middle Aptian. Nannoconids are the main carbonate producers in both limestones and marls. Because of their low content of diagenetic minerals (such as ankerite), the trace-element records of the bulk carbonate closely approach those of Nannoconus
spp. so geochemical sequences can be defined. The long-term evolution of Sr and Mn content is linked to fluctuations in the chemistry of sea-water due to differences in the rate of their supply by rivers and hydrothermal sources, to changes in sea level and to variation in the ratio of aragonite to calcite production on the platforms. Diagenetic processes are not involved, nor are variations in carbonate mineralogy or changes in the number or nature of pelagic carbonate producers in the hemipelagic sediments of La Marcouline quarry.
![]() We are grateful to the reviewers H. and K.B. for their helpful constructive criticism of the manuscript. Thanks are due to Nestor for corrections of syntax and word choice that improved the readability of the text. We would also like to thank Nathalie (Laboratoire Biominéralisations et Paleoenvironnements, UPMC) for her efficient technical assistance, and W. , J.-P. and J.M for their help during field work.
We are grateful to the reviewers H. and K.B. for their helpful constructive criticism of the manuscript. Thanks are due to Nestor for corrections of syntax and word choice that improved the readability of the text. We would also like to thank Nathalie (Laboratoire Biominéralisations et Paleoenvironnements, UPMC) for her efficient technical assistance, and W. , J.-P. and J.M for their help during field work.
H., M., J.F., B. & J.J. (1989).- Géochimie des carbonates (Mn, Sr) et minéralogie des argiles de calcaires pélagiques sénoniens. Relations avec les variations eustatiques (Massif de la Maiella, Abruzzes, Italie).- Comptes-Rendus de l'Académie des Sciences, Paris, (II), t. 309, p. 1679-1685.
H., M. & N.O. (1993).- Le manganèse dans les carbonates pélagiques : Un outil d'intérêt stratigraphique et paléogéographique (le Sénonien d'Italie, de Tunisie et du Danemark).- Comptes-Rendus de l'Académie des Sciences, Paris, (II), t. 316, p. 1-8.
A., H. & T. (2006).- Sr / Ca ratios as indicators of varying modes of pelagic carbonate diagenesis in the ooze, chalk and limestone realms.- Sedimentary Geology, Amsterdam, vol. 191, n° 1-2, p. 37-53.
B., A., S., P., N. & H. (2005).- Coccolith chemistry as proxy of coccolithophorid productivity response to monsoon cycles.- EOS, Transactions of the American Geophysical Union, Washington D.C., 86(52), Fall Meeting Suppl., Abstract OS51A-0550.
D.J., M.R. & D.R. (2002).- On the nature of methane gas-hydrate dissociation during the Toarcian and Aptian oceanic anoxic events.- American Journal of Science, New Haven, vol. 302, p. 28-49.
C. (2006).- Variations des paramètres de l'environnement océanique au cours de la sédimentation d'un doublet marne-calcaire. Approches géochimique, minéralogique et micropaléontologique.- Thèse de Doctorat, Mémoires des Sciences de la Terre, Université Pierre et Marie , Paris, n° 2006-05, 252 p.
C., M. de, M., M. & G. (2007).- Environmental changes during marl-limestone formation: Evidence from the Gargasian (Middle Aptian) of La Marcouline Quarry (Cassis, SE France).- Carnets de Géologie / Notebooks on Geology, Brest, Article 2007/01 (CG2007_A01), 13 p.
K., R.E.M. & D.P. (2004).- Cenozoic pelagic Sr/Ca records: Exploring a link to paleoproductivity.- Paleoceanography, Washington D.C., vol. 19, part 3, p. PA3005.
U. (1981).- Mineralogy and chemistry of the lower Pennsylvanian Kendrick fauna, eastern Kentucky: 1. Trace elements.- Chemical Geology, Amsterdam, vol. 32, n° 1-4, p. 1-16.
H.E. & R.M. (1983).- Diagenesis of deep-sea carbonates. In: G. & G.V. (eds.), Diagenesis in sediments and sedimentary rocks. Volume 2.- Development in Sedimentology, Elsevier, Amsterdam, 25B, p. 213-288.
J.C., A., A., B. & M. (2000).- Manganese in pelagic carbonates: Indication of major tectonic events during the geodynamic evolution of a passive continental margin (the Jurassic European Margin of the Tethys-Ligurian Sea).- Palaeogeography, Palaeoclimatology, Palaeoecology, Amsterdam, vol. 156, n° 1-2, p. 123-138.
H.G. & B.A. (1981).- Climatically controlled variation of Sr and Mg in Quaternary planktonic foraminifera.- Nature, London, vol. 291, p. 61-64.
L. (1993).- Apport de la géochimie à la stratigraphie séquentielle. Application au Crétacé inférieur vocontien.- Mémoires des Sciences de la Terre, Université Pierre et Marie , Paris, n° 93-5, 191 p.
L. & M. (1993).- Carbonate geochemistry (Mn, δ13C, δ18O) of the Late Tithonian-Berriasian pelagic limestones of the Vocontian Trough (SE France).- Bulletin des Centres de Recherches Exploration-Production elf-Aquitaine, Pau, vol. 17, n° 1, p. 205-221.
E. (1992).- Calcareous nannofossil distribution in pelagic rhythmic sediments (Aptian-Albian Piobbico core, central Italy).- Rivista italiana di Paleontologia e Stratigrafia, Milano, vol. 97, n° 3-4, p. 455-484.
T.D., M.A. & W.E. (1999).- Diagenesis of Lower Cretaceous pelagic carbonates, North Atlantic; paleoceanographic signals obscured.- The Journal of Foraminiferal Research, Washington D.C., vol. 29, n° 4, p. 340-351.
H., K.B. & H. (1993).- Evolution of the Tithonian-Aptian carbonate platform along the northern Tethyan margin, eastern Helvetic Alps. In: T., R.W. & J.P. (eds.), Cretaceous carbonate platforms.- American Association of Petroleum Geologists, Memoir, Tulsa, 56, p. 387–407.
D.W., M.L., D.F. & L.D. Jr (1982).- Strontium-calcium ratios in Cenozoic planktonic foraminifera.- Geochimica et Cosmochimica Acta, Oxford, vol. 46, n° 7, p. 1281-1292.
J., J., M.B., T., P.C. de & P.R. (1998).- Cretaceous sequence chronostratigraphy. In: P.C. de, J., T., P.R. & M.B. (eds.), Mesozoic and Cenozoic sequence stratigraphy of European basins.- Society of Economic Paleontologists and Mineralogists, Special Publication, Tulsa, vol. 60, Chart 4.
H.D., H.J. & J.L. (1964).- The coprecipitation of cations with CaCO3- II. The coprecipitation of Sr2+ with calcite between 90° and 100°C.- Geochimica et Cosmochimica Acta, Oxford, vol. 28, n° 8, p. 1287-1301.
A.H., N.C., G., J. & R. (2001).- Terrestrial record of methane hydrate dissociation in the Early Cretaceous.- Geology, Boulder, vol. 29, n° 2, p. 159-162.
I., A.M. & A.S. (2001).- Geochemistry of pelagic and hemipelagic carbonates: criteria for identifying systems tracts and sea-level change.- Journal of the Geological Society, London, vol. 158, n° 4, p. 685-696.
C.E. & H.C. (2002).- Seawater Sr isotopes, oceanic anoxic events, and sea floor hydrothermal activity in the Jurassic and Cretaceous.- American Journal of Science, New Haven, vol. 301, p. 112-149.
W. & M. (2007).- The Gargasian (Middle Aptian) of La Marcouline section at Cassis-La Bédoule (S.E France): Stable Isotope record and orbital cyclicity.- Carnets de Géologie / Notebooks on Geology, Brest, Article 2007/02 (CG2007_A02), 9 p.
J.J.D. (1969).- Interpretation of Sr2+ concentrations in carbonate minerals and rocks.- Journal of Sedimentary Research, Tulsa, vol. 39, n° 2, p. 486-508.
Y., A. & T. (1979).- Magnesian calcite synthesis from calcium bicarbonate solution containing magnesium and barium ions.- Geochemical Journal, Kyushu, vol. 13, n° 4, p. 181-185.
G., N., G., U., A., H., B., S. & S. (2006).- Coccolith strontium to calcium ratios in Emiliania huxleyi: The dependence on seawater strontium and calcium concentrations.- Limnology and Oceanography, Canmore, vol. 51, n° 1, p. 310-320.
D. (2002).- Enregistrement à haute fréquence des conditions environnementales par les tests de bivalves. Applications des techniques de marquage, cathodoluminescence et chimie à l'huître creuse Crassostrea gigas de l'étang de Thau (Hérault, France).- Thèse de Doctorat, Mémoires des Sciences de la Terre, Université Pierre et Marie , Paris, n° 2002-05, 231 p.
D., M., M. de, M., M., E. & D. (2006).- Experimental and natural cathodoluminescence in the shell of Crassostrea gigas from the Thau lagoon (France): Ecological and environmental implications.- Marine Ecology, Progress series, Oldendorf/Luhe, vol. 317, p. 143-156.
R.B. & M.L. (1980).- The impact of solution chemistry on Mytilus edulis calcite and aragonite.- Geochimica et Cosmochimica Acta, Oxford, vol. 44, n° 9, p. 1265-1278.
F.T. & J.D. (1981).- Tectonic controls of Phanerozoic sedimentary rock cycling.- Journal of the Geological Society, London, vol. 138, n° 2, p. 183-196.
J.P. & J. (1981).- Cretaceous coral-rudist buildups in France. In: D.F. (ed.), European fossil reef models.- Society of Economic Paleontologists and Mineralogists, Special Publication, Tulsa, vol. 30, p. 399-426.
E. & B. (2002).- Contribution of calcareous nannoplankton to carbonate deposition: A new approach applied to the Lower Jurassic of central Italy.- Marine Micropaleontology, Amsterdam, vol. 45, n° 2, p. 175-190.
F., M. de, M., S. & J. (2005).- Changes in the pelagic fine fraction carbonate sedimentation during the Cretaceous–Paleocene transition: Contribution of the separation technique to the study of Bidart section.- Palaeogeography, Palaeoclimatology, Palaeoecology, Amsterdam, vol. 216, n° 1-2, p. 119-137.
C.H. (2001).- Carbonate reservoirs, porosity evolution and diagenesis in a sequence stratigraphic framework.- Development in Sedimentology, Elsevier, New York, 55, 444 p.
J.W, Q. & M.Y. (1997).- Influence of temperature and Mg/Ca ratio on CaCO3 precipitates from sea-water.- Geology, Boulder, vol. 25, n° 1, p. 85-87.
M., J.-P. & G. (2002).- Hierarchy of criteria, evolutionary processes and taxonomic simplification in the classification of Lower Cretaceous planktonic foraminifera.- Cretaceous Research, Oxford, vol. 23, n° 1, p. 111-148.
M., W., J.A., J.-P. & G. (1998).- Correlation of biostratigraphic and stable isotope events in the Aptian historical stratotype of La Bédoule (southeast France).- Comptes Rendus de l'Académie des Sciences, Paris, (Série IIa, Sciences de la Terre et des Planètes), t. 327, p. 693-698.
M. & G. (2004).- The Gargasian (Middle Aptian) substage in the Aptian historical stratotypes (SE France): General introduction.- Carnets de Géologie / Notebooks on Geology, Maintenon, Letter 2004/01 (CG2004_L01), 3 p.
M., G. & J.-P. (2005).- Le Gargasien (Aptien moyen) de Cassis-la Bédoule (stratotype historique de l'Aptien inférieur, SE France) : associations et biostratigraphie des Foraminifères benthiques et planctoniques.- Carnets de Géologie / Notebooks on Geology, Brest, Article 2005/02 (CG2005_A02), 20 p.
M., G., W., M. & J.-P. (2004).- Le Gargasien (Aptien moyen) de Cassis-La Bédoule (stratotype historique de l'Aptien inférieur, SE France) : Localisation géographique et corrélations stratigraphiques.- Carnets de Géologie / Notebooks on Geology, Maintenon, Letter 2004/02 (CG2004_L02), 4 p.
D., G., A., Y. & A.M. (1994).- Contribution fondamentale des Coccolithophoridées à la constitution des calcaires fins pélagiques du Jurassique moyen et supérieur.- In: 3e Symposium international sur la Stratigraphie du Jurassique, Poitiers, 1991.- Géobios, Lyon, vol. 17, p. 701-721.
B. (1983).- Geochemistry of the Late Cenomanian-Early Turonian chalks of the Paris Basin: Manganese and carbon isotopes in carbonates as paleooceanographic indicators.- Cretaceous Research, Oxford, vol. 4, p. 85-93.
M. de (2000).- Apport de l'étude de la spéciation du manganèse dans les carbonates pélagiques à la compréhension du contrôle des séquences eustatiques de 3ème ordre.- Mémoires des Sciences de la Terre, Université Pierre et Marie , Paris, n° 2000-02, 214 p.
M. de, L., M., F. & R. (2001).- Geochemical characterisation (Mn content) of third order sequences in Upper Jurassic pelagic carbonates of the Vocontian Trough (S.E. France).- Eclogae Geologicae Helvetiae, Basel, vol. 94, p. 145-152.
M. de, M., L. & C. (2000).- Apport de la cathodoluminescence à la connaissance de la spéciation du manganèse dans les carbonates pélagiques.- Comptes-Rendus de l'Académie des Sciences, Paris, (II), t. 330, p. 391-398.
R., P.K., K. & K. (2005).- Magnesium and strontium compositions of recent symbiont-bearing benthic foraminifera.- Marine Micropaleontology, Amsterdam, vol. 58, n° 1, p. 31-44
M. & P. (1971).- Mise au point d'un protocole expérimental pour le dosage d'éléments en traces (V, Cr, Mn, Ni, Sr, Mo) par absorption atomique.- Comptes-Rendus de l'Académie des Sciences, Paris, (II), t. 272, p. 2285-2288.
M. & P. (1972).- Influence des conditions de mise en solution (choix de l'acide, température, durée d'attaque) dans le dosage des éléments en traces des roches carbonatées.- Comptes-Rendus de l'Académie des Sciences, Paris, (II), t. 274, p. 632-635.
M., M. de, M., M., J.P., W., J.A. & G. (2005).- Early Aptian δ13C and manganese anomalies from the historical Cassis-La Bédoule stratotype sections (S.E. France): Relationship with a methane hydrate dissociation event and stratigraphic implications.- Carnets de Géologie / Notebooks on Geology, Brest, Article 2005/04 (CG2005_A04), 18 p.
M. & M. de (1998).- Géochime des éléments traces de la phase carbonatée des calcaires de la coupe du stratotype historique de l'Aptien inférieur dans la région de Cassis-La Bédoule (S.E. France).- Géologie Méditerranéenne, Marseille, t. XXV, n° 3-4, p. 43-54.
M. (1985).- Géochimie des carbonates pélagiques : Mise en évidence des fluctuations de la composition des eaux océaniques depuis 140 MA. Essai de chimiostratigraphie.- Documents du BRGM, Orléans, n° 85, 650 p.
M. (1986).- Pelagic carbonate chemostratigraphy (Sr, Mg, 18O, 13C).- Marine Micropaleontology, Amsterdam, vol. 10, n° 1-3, p. 117-164.
G. (1990).- Dosage de quelques éléments traces dans les eaux naturelles et les roches carbonatées. Application à l'étude géochimique de la coupe du Kef (Tunisie).- Diplôme d'Études Supérieures, Université Pierre et Marie , Paris, 177 p.
F.M & Y. (1993).- The rate and consequences of Sr diagenesis in deep-sea carbonates.- Earth and Planetary Science Letters, Amsterdam, vol. 117, p. 553-565.
F. & M. (1995).- Foraminifères planctoniques du Crétacé : Commentaire de la zonation Europe-Méditerranée.- Bulletin de la Société Géologique de France, Paris, t. 166, n° 6, p. 681-692.
P.A. (1975).- New interpretations of Great Salt Lake ooids and of ancient non-skeletal carbonate mineralogy.- Sedimentology, Oxford, vol. 22, n° 4, p. 497-537.
S.M. & L.A. (1998).- Secular oscillations in the carbonate mineralogy of reef-building and sediment-producing organisms driven by tectonically forced shifts in seawater chemistry.- Palaeogeography, Palaeoclimatology, Palaeoecology, Amsterdam, vol. 144, n° 1-2, p. 3-19.
S.M. (2006).- Influence of seawater chemistry on biomineralization throughout Phanerozoic time: Paleontological and experimental evidence.- Palaeogeography, Palaeoclimatology, Palaeoecology, Amsterdam, vol. 232, n° 2-4, p. 214-236.
T. (1999).- Isotopic and chemical intra-shell variations in low-Mg calcite of rudist bivalves (Mollusca-Hippuritacea): Disequilibrium fractionations and Late Cretaceous seasonality.- International Journal of Earth Sciences, Berlin, vol. 88, n° 3, p. 551-570.
T. & J. (2002).- Phanerozoic record of plate tectonic control of seawater chemistry and carbonate sedimentation.- Geology, Boulder, vol. 30, n° 12, p. 1123-1126.
H.M. (2003).- Coccolith chemistry as a paleoceanographic indicator in Paleogene and Neogene sediments.- EOS, Transactions of the American Geophysical Union, Washington D.C., Fall Meeting 2003, Abstract PP52B-06.
H.M., D.P. & S.C. (1999).- Are seawater Sr/Ca variations preserved in Quaternary foraminifera?- Geochimica et Cosmochimica Acta, Oxford, vol. 63, n° 21, p. 3535-3547.
H.M. & D.P. (2001).- Sr/Ca variations in Cretaceous carbonates: Relation to productivity and sea level changes.- Palaeogeography, Palaeoclimatology, Palaeoecology, Amsterdam, vol. 168, p. 311-336.
H.M., C.M., I., J.R. & J.I. (2002).- Calcification rate and temperature effects on Sr partitioning in coccoliths of multiple species of coccolithophorids in culture.- Global and Planetary Change, Amsterdam, vol. 34, n° 3-4, p. 153-171.
H.M. & S. (2003).- Coccolith Sr/Ca records of productivity during the Paleocene-Eocene thermal maximum from the Wedell Sea.- Paleoceanography, Washington D.C., vol. 18, n° 2, p. 1049.
H.M. (2005).- Limited range of interspecific vital effects in coccolith stable isotopic records during the Paleocene Eocene thermal maximum.- Paleoceanography, Washington D.C., vol. 20, n° 1, Art. No. PA1007.
J., R. & J. (1971).- Possible use of strontium in sedimentary carbonate rocks as a paleoenvironmental indicator.- Sedimentary Geology, Amsterdam, vol. 5, n° 1, p. 5-23.
J. (1978).- Simulation of limestone diagenesis. A model based on strontium depletion: Discussion.- Canadian Journal of Earth Sciences, Ottawa, vol. 15, n° 10, p. 1683-1685.
J. (1983).- Chemical diagenesis of carbonates: Theory and application of trace element technique in stable isotope. In: M.A., T.T., I.R., L.S. & J. (eds.), Sedimentary geology.- Society of Economic Paleontologists and Mineralogists, Short Course Notes, Tulsa, n° 10, 151 p.
A., L., S.J., R. & K. (2005).- Geochemical support for insolation linked Nannofossil productivity changes during the Mid-Pliocene.- EOS, Transactions of the American Geophysical Union, Washington D.C., 86(52), Fall Meeting Suppl., Abstract PP51B-0586.
H., A., K.B. & O. (1998).- Correlation of Early Cretaceous carbon isotope stratigraphy and platform drowning events: A possible link?- Palaeogeography, Palaeoclimatology, Palaeoecology, Amsterdam, vol. 137, n° 3-4, p. 189-203.
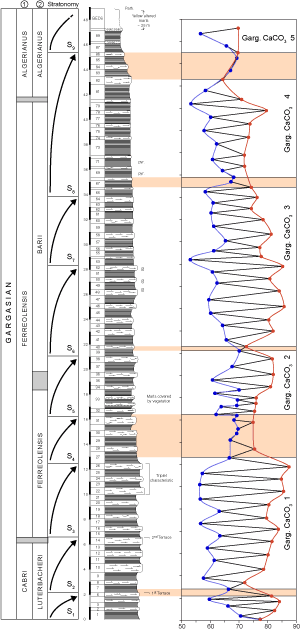
Click on thumbnail to enlarge the image.
Figure 1: Lithology, sequence pattern and CaCO3 content in the Gargasian beds of La Marcouline quarry. Blue line and circles: CaCO3 contents in the marls (soft beds) ; red line and circles: CaCO3 contents in the limestones (hard beds). Biostratigraphic scales from (1) & (1995) and et alii (2002) and (2) from et alii (2005).
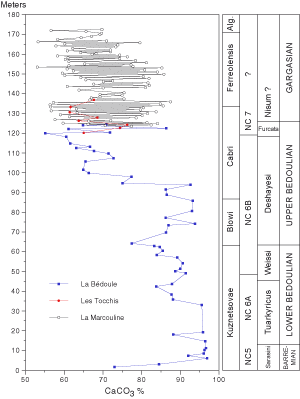
Click on thumbnail to enlarge the image.
Figure 2: Evolution in the CaCO3 content of Bedoulian and Gargasian sediments from the Cassis area (La Bédoule, Les Tocchis and La Marcouline).
| Structureless carbonate particles of uncertain origin | Calcareous Nannofossils | ||||||||
| Ankerite macro- particles |
Calcite macro- particles |
Micarbs | Coccoliths | Nannoconus sp. | |||||
| Marls | 2.5 | 6 | 58 | 8 | 25.5 | ||||
| Watznaueria sp. | Rhagodiscus sp. | Biscutum sp. | Others | Fragments | |||||
| 1.2 | 1 | 0.1 | 0.2 | 5.5 | |||||
| Limestones | 2.5 | 6 | 63 | 8.5 | 20 | ||||
| Watznaueria sp. | Rhagodiscus sp. | Biscutum sp. | Others | Fragments | |||||
| 1.8 | 0.7 | 0.1 | 0.1 | 6 | |||||
Click on thumbnail to enlarge the image.
Figure 3: Mean composition of carbonate fraction in marls and limestones (volume %, from , 2006).
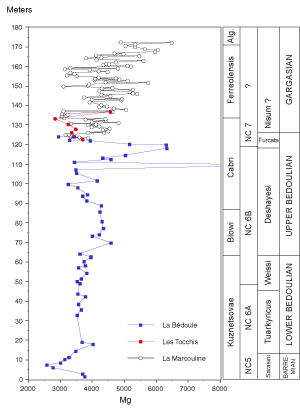
Click on thumbnail to enlarge the image.
Figure 4: Evolution of Mg content in bulk carbonate in Bedoulian to Gargasian sediments from the Cassis area (La Bédoule, Les Tocchis and La Marcouline). For outcrop locations see et alii (2004).
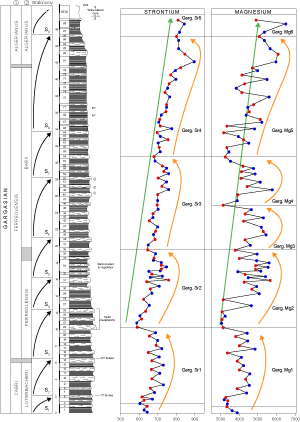
Click on thumbnail to enlarge the image.
Figure 5: Sr and Mg contents in the bulk carbonate of Gargasian beds in the La Marcouline quarry. Blue circles: Marls (soft beds); red circles: Limestones (hard beds).
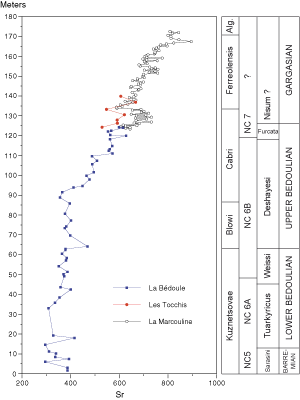
Click on thumbnail to enlarge the image.
Figure 6: Evolution of Sr content in bulk carbonate in Bedoulian to Gargasian sediments from the Cassis area (La Bédoule, Les Tocchis and La Marcouline).
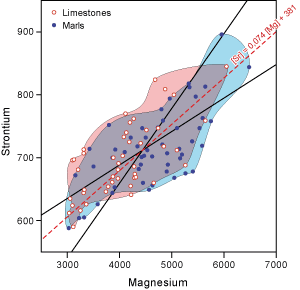
Click on thumbnail to enlarge the image.
Figure 7: Relationship between the Sr and Mg content of Gargasian beds in La Marcouline quarry. Blue circles: Marls (soft beds); red circles: Limestones (hard beds).
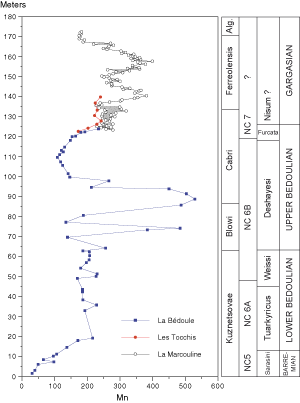
Click on thumbnail to enlarge the image.
Figure 8: Evolution of bulk carbonate Mn content in Bedoulian to Gargasian sediments from the Cassis area (La Bédoule, Les Tocchis and La Marcouline).
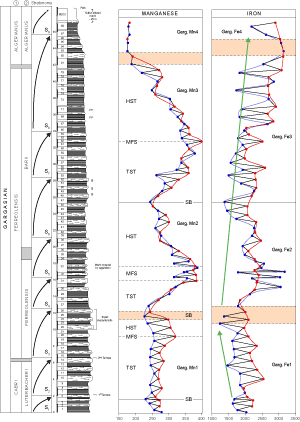
Click on thumbnail to enlarge the image.
Figure 9: Mn and Fe content of bulk carbonates in the Gargasian beds of La Marcouline quarry. Blue line and circles: Marls (soft beds); red line and circles: Limestones (hard beds).
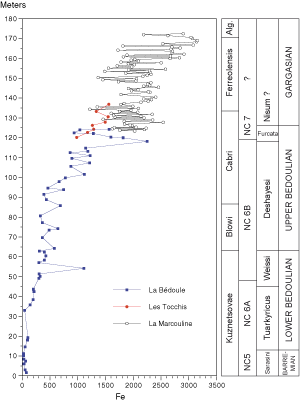
Click on thumbnail to enlarge the image.
Figure 10: Evolution of Fe in the bulk carbonate of Bedoulian to Gargasian sediments from the Cassis area (La Bédoule, Les Tocchis and La Marcouline).
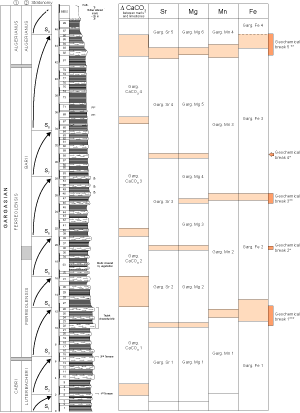
Click on thumbnail to enlarge the image.
Figure 11: Schematic pattern of the chemostratigraphic zonation of the La Marcouline Gargasian section. Comparison with the stratonomy (1) and the biostratigraphic zonations (2) - (1) from & (1995) and et alii (2002) and (2) from et alii (2005).
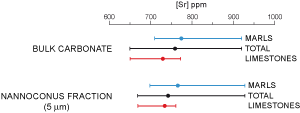
Click on thumbnail to enlarge the image.
Figure 12: Comparison of the content of Sr in bulk carbonates and the Sr content of the 5 µm fraction in beds 29 to 32. The 5 µm fraction is mainly Nannoconus (up to 80%). Neither cementation nor diagenesis appears to have played a significant role. The Sr content of limestones is lower than that of marls in both bulk carbonate and in the Nannoconus fraction. Consequently, this relationship cannot be attributed to lithologic factors that might influence diagenesis.
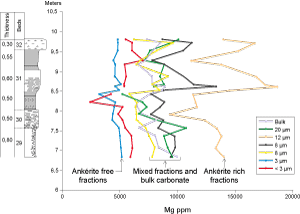
Click on thumbnail to enlarge the image.
Figure 13: Comparison of the Mg content of bulk carbonate in beds 29 to 32 with that of various granulometric fractions. The fraction 12 µm concentrates ankerite macroparticles. Only fine fractions are more or less ankerite-free.
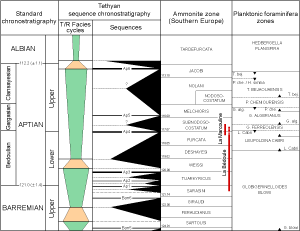
Click on thumbnail to enlarge the image.
Figure 14: Chronostratigraphic chart for the Aptian from et alii (1998): Location of 2nd and 3rd order sequences relative to ammonitic and planktonic foraminiferal zones.
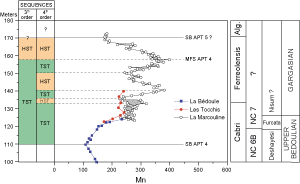
Click on thumbnail to enlarge the image.
Figure 15: Evolution of bulk carbonate Mn during the late Bedoulian and early Gargasian. Relationship with the Aptian 4 eustatic sequence of et alii (1998). SB = sequence boundary, TST = transgressive system tract, MFS = maximum flooding surface and HST = highstand system tract.
| Bed | Echantillons | Lithology | CaCO3 | Ca | Mg | Sr | Mn | Fe |
| 1 | MAR.1 | marl | 77.41 | 402269 | 3862 | 644 | 278 | 2027 |
| 1 | MAR.2 | marl | 70.60 | 389243 | 3581 | 626 | 257 | 1759 |
| 2 | MAR.3 | limestone | 82.44 | 413993 | 3312 | 644 | 272 | 2202 |
| 3 | MAR.4 | marl | 66.17 | 392512 | 3321 | 605 | 242 | 1623 |
| 4 | MAR.5 | limestone | 84.10 | 400189 | 3362 | 626 | 272 | 2233 |
| 5 | MAR.6 | marl | 59.71 | 390936 | 3154 | 672 | 229 | 1660 |
| 6 | MAR.7 | limestone | 81.38 | 387065 | 3251 | 616 | 259 | 2051 |
| 7 | MAR.8 | marl | 66.36 | 389249 | 4367 | 682 | 248 | 1980 |
| 8 | MAR.9 | limestone | 72.03 | 385941 | 3859 | 661 | 278 | 1945 |
| 9 | MAR.10 | marl | 57.63 | 389408 | 4500 | 732 | 244 | 1843 |
| 10 | MAR.11 | limestone | 78.59 | 399268 | 4312 | 669 | 268 | 2539 |
| 11 | MAR.12 | marl | 61.38 | 396504 | 4531 | 712 | 256 | 1837 |
| 12 | MAR.13 | limestone | 80.14 | 396924 | 3969 | 667 | 271 | 2350 |
| 13 | MAR.14 | marl | 59.02 | 392785 | 4214 | 732 | 246 | 1458 |
| 14 | MAR.15 | limestone | 81.52 | 403453 | 3873 | 670 | 284 | 2195 |
| 15 | MAR.16 | marl | 63.35 | 393347 | 4099 | 709 | 244 | 1671 |
| 16 | MAR.17 | limestone | 83.75 | 395846 | 3325 | 649 | 272 | 2138 |
| 17 | MAR.18 | marl | 56.65 | 388558 | 4818 | 721 | 246 | 1647 |
| 18 | MAR.19 | limestone | 79.66 | 398004 | 3980 | 691 | 287 | 2340 |
| 19 | MAR.20 | marl | 63.12 | 382975 | 4422 | 701 | 256 | 1603 |
| 20 | MAR.21 | limestone | 80.42 | 411078 | 3782 | 654 | 319 | 2080 |
| 21 | MAR.22 | marl | 56.52 | 393486 | 4407 | 689 | 253 | 1755 |
| 22 | MAR.23 | limestone | 85.57 | 389148 | 3113 | 590 | 304 | 1961 |
| 23 | MAR.24 | marl | 56.35 | 398327 | 3027 | 588 | 236 | 1219 |
| 24 | MAR.25 | limestone | 84.82 | 399297 | 3035 | 612 | 299 | 2092 |
| 25 | MAR.26 | marl | 57.25 | 383978 | 3225 | 604 | 227 | 1352 |
| 26 | MAR.27 | limestone | 87.46 | 401760 | 3053 | 635 | 244 | 1993 |
| 27 | MAR.28 | marl | 66.65 | 385305 | 4630 | 656 | 239 | 1777 |
| 28 | MAR.29 | limestone | 75.45 | 387194 | 3098 | 624 | 268 | 1835 |
| 29 | MAR.30 | marl | 67.00 | 380048 | 5048 | 668 | 301 | 2138 |
| 30 | MAR.31 | limestone | 69.26 | 401177 | 4654 | 660 | 306 | 2271 |
| 31 | MAR.32 | marl | 74.94 | 390698 | 4220 | 641 | 344 | 2329 |
| 31 | MAR.33 | marl | 69.27 | 382298 | 4447 | 660 | 354 | 2188 |
| 32 | MAR.34 | limestone | 75.47 | 396647 | 4284 | 668 | 381 | 2404 |
| 33 | MAR.35 | marl | 63.83 | 393504 | 3904 | 653 | 345 | 1780 |
| 33 | MAR.36 | marl | 70.06 | 395392 | 4903 | 678 | 359 | 2301 |
| 34 | MAR.37 | limestone | 81.01 | 396952 | 3970 | 681 | 364 | 2271 |
| 35 | MAR.38 | marl | 60.80 | 381648 | 4924 | 689 | 310 | 1970 |
| 36 | MAR.39 | limestone | 81.98 | 389342 | 3738 | 646 | 330 | 2087 |
| 37 | MAR.40 | marl | 67.63 | 385691 | 4564 | 649 | 306 | 1914 |
| 38 | MAR.41 | limestone | 81.64 | 396100 | 4278 | 686 | 274 | 2456 |
| 39 | MAR.42 | marl | 70.11 | 389537 | 5402 | 678 | 276 | 2189 |
| 40 | MAR.43 | limestone | 72.54 | 398431 | 5259 | 688 | 274 | 2295 |
| 41 | MAR.44 | marl | 65.59 | 375455 | 5158 | 699 | 259 | 2260 |
| 42 | MAR.45 | limestone | 81.92 | 390028 | 4212 | 655 | 300 | 2317 |
| 43 | MAR.46 | marl | 64.30 | 381402 | 5261 | 675 | 271 | 1733 |
| 44 | MAR.47 | limestone | 80.42 | 397367 | 4292 | 674 | 291 | 2050 |
| 45 | MAR.48 | marl | 60.15 | 382202 | 4623 | 702 | 267 | 1453 |
| 46 | MAR.49 | limestone | 85.76 | 387303 | 3098 | 696 | 251 | 2060 |
| 47 | MAR.50 | marl | 59.52 | 387974 | 3863 | 700 | 244 | 1369 |
| 48 | MAR.51 | limestone | 84.05 | 387977 | 3880 | 700 | 267 | 2173 |
| 49 | MAR.52 | marl | 62.36 | 411943 | 5748 | 758 | 279 | 2251 |
| 50 | MAR.53 | limestone | 80.79 | 393196 | 4404 | 723 | 308 | 2320 |
| 51 | MAR.54 | marl | 60.76 | 392205 | 5098 | 747 | 288 | 1866 |
| 52 | MAR.55 | limestone | 85.24 | 390380 | 4060 | 703 | 329 | 2303 |
| 53 | MAR.56 | marl | 53.05 | 389568 | 4831 | 759 | 262 | 1488 |
| 54 | MAR.57 | limestone | 77.77 | 392509 | 4082 | 733 | 360 | 2292 |
| 55 | MAR.58 | marl | 61.21 | 392525 | 4733 | 748 | 323 | 1918 |
| 56 | MAR.59 | limestone | 77.28 | 387537 | 4185 | 725 | 358 | 2581 |
| 57 | MAR.60 | marl | 65.31 | 390411 | 3501 | 686 | 338 | 1553 |
| 58 | MAR.61 | limestone | 81.24 | 400759 | 3206 | 681 | 338 | 1972 |
| 59 | MAR.62 | marl | 61.21 | 423052 | 3424 | 714 | 378 | 1712 |
| 60 | MAR.63 | limestone | 78.42 | 394436 | 3313 | 707 | 361 | 2020 |
| 61 | MAR.64 | marl | 60.29 | 388117 | 4469 | 703 | 354 | 1912 |
| 62 | MAR.65 | limestone | 74.34 | 403756 | 4199 | 756 | 399 | 2503 |
| 63 | MAR.66 | marl | 60.99 | 390893 | 3917 | 713 | 339 | 1779 |
| 64 | MAR.67 | limestone | 76.32 | 390707 | 3126 | 697 | 363 | 2094 |
| 65 | MAR.68 | marl | 58.28 | 390947 | 4801 | 777 | 334 | 1963 |
| 66 | MAR.69 | limestone | 74.34 | 394561 | 3314 | 713 | 360 | 2028 |
| 67 | MAR.70 | marl | 67.21 | 383645 | 5194 | 705 | 347 | 2664 |
| 68 | MAR.71 | limestone | 68.25 | 385904 | 5097 | 712 | 338 | 2832 |
| 69 | MAR.72 | marl | 64.28 | 387657 | 4342 | 718 | 326 | 1822 |
| 70 | MAR.73 | limestone | 71.96 | 389180 | 4514 | 744 | 342 | 2670 |
| 71 | MAR.74 | marl | 60.80 | 392382 | 4433 | 747 | 305 | 1888 |
| 72 | MAR.75 | limestone | 71.91 | 394810 | 4738 | 747 | 317 | 2914 |
| 73 | MAR.76 | marl | 62.21 | 386520 | 5590 | 763 | 303 | 2699 |
| 74 | MAR.77 | limestone | 73.33 | 396977 | 4129 | 740 | 260 | 2707 |
| 75 | MAR.78 | marl | 57.86 | 379660 | 3797 | 752 | 249 | 1724 |
| 76 | MAR.79 | limestone | 73.85 | 395465 | 4271 | 762 | 264 | 2681 |
| 77 | MAR.80 | marl | 60.15 | 386142 | 5457 | 797 | 266 | 2844 |
| 78 | MAR.81 | limestone | 79.56 | 395477 | 4113 | 770 | 280 | 2697 |
| 79 | MAR.82 | marl | 53.26 | 387535 | 4960 | 794 | 219 | 1814 |
| 80 | MAR.83 | limestone | 70.94 | 390031 | 4680 | 824 | 271 | 3081 |
| 81 | MAR.84 | marl | 58.32 | 386971 | 5953 | 896 | 187 | 2534 |
| 82 | MAR.85 | limestone | 64.37 | 384437 | 6046 | 845 | 191 | 3139 |
| 83 | MAR.86 | marl | 67.06 | 389581 | 5640 | 813 | 175 | 3154 |
| 84 | ||||||||
| 85 | MAR.87 | marl | 69.26 | 385892 | 5338 | 812 | 177 | 3065 |
| 86 | MAR.88 | limestone | 69.66 | 393814 | 5041 | 800 | 175 | 3040 |
| 87 | ||||||||
| 88 | MAR.89 | marl | 65.64 | 384791 | 5323 | 818 | 181 | 2624 |
| 89 | MAR.90 | marl | 56.61 | 368112 | 6479 | 844 | 178 | 2187 |
| 90 | MAR.91 | marl | 69.75 | 392938 | 4872 | 809 | 182 | 2900 |
Click on thumbnail to enlarge the image.
Appendix 1: Trace element contents of bulk carbonates from La Marcouline Quarry.